Ligand for Target Protein refers to a molecule that specifically binds to a target protein. A target protein ligand interacts specifically with the target protein through non-covalent bonds such as hydrogen bonds, ionic bonds, van der Waals forces, and covalent bonds. This interaction has a certain affinity and selectivity, making it a key element in protein function research, drug development, and other fields.
Types of Target Protein Ligands
- Small Molecule Compounds: These have small molecular weight, are easy to synthesize and modify, and can easily cross the cell membrane to reach intracellular target proteins. For example, certain kinase inhibitors bind to specific kinase target proteins to inhibit their activity and interfere with tumor cell proliferation.
- Biomacromolecules: These include proteins, peptides, and nucleic acids. Antibodies are a typical example of biomacromolecule target protein ligands that specifically bind to antigen target proteins, playing an important role in immune responses, disease diagnosis, and therapy.
- Other Ligands: Special ligands, such as certain metal ions or small peptides, can also bind to specific target proteins and exert effects in particular physiological or pathological processes.
Applications in Biomedical Fields
Basic Research: It can be used to study the relationship between protein function, structure, and activity. By observing changes after the target protein ligand binds to the target protein, we can gain insights into the mechanism of protein action in cellular physiological processes.
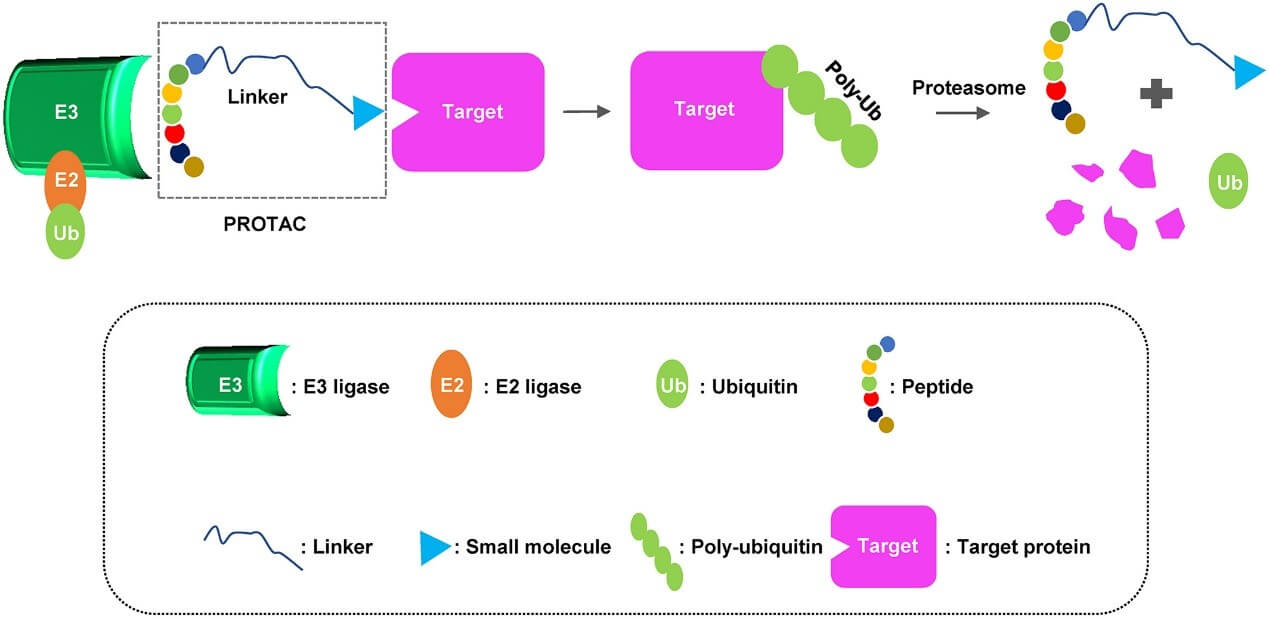
Drug Development: It forms the essential basis for drug design. Many drugs are essentially target protein ligands, such as inhibitors and agonists, which regulate the activity or function of the target protein to treat diseases. For example, in PROTAC technology, the target protein ligand guides the E3 ligase to bind to the target protein, leading to ubiquitination and degradation by the proteasome.
Role of Target Protein Ligands in PROTAC
Inducing Target Protein Proximity to E3 Ubiquitin Ligase: One end of a PROTAC molecule is a target protein ligand, and the other end is an E3 ubiquitin ligase ligand. After entering the cell, the target protein ligand binds to the target protein, and the E3 ligase ligand binds to the E3 ligase, bringing the target protein and the E3 ligase close enough to allow for ubiquitination.
Promoting Target Protein Ubiquitination: Under the combined action of the target protein ligand and the E3 ligase ligand, the E3 ligase is recruited to the target protein, where it recognizes and catalyzes the transfer of ubiquitin molecules to the lysine residues of the target protein. As more ubiquitin molecules are added, a polyubiquitin chain is formed, which marks the target protein for degradation.
Leading to Target Protein Degradation: The polyubiquitinated target protein is recognized and bound by the 26S proteasome. The proteasome removes the ubiquitin chains through its deubiquitinating enzymes, then uses its protease activity to degrade the target protein into small peptide fragments and amino acids. These degradation products can be reused by the cell for protein synthesis and other metabolic processes, resulting in specific degradation of the target protein.
Improving the Degradation Efficiency and Selectivity of PROTAC Molecules: The specificity and affinity of target protein ligands directly affect the binding efficiency of PROTAC molecules to the target protein. High specificity and high affinity target protein ligands allow PROTAC to more accurately recognize and bind to the target protein, reducing non-specific binding to other proteins and thus enhancing the selectivity of PROTAC molecules. Additionally, suitable target protein ligands help form stable ternary complexes, improving the ubiquitination efficiency of the target protein by the E3 ligase and, in turn, increasing the degradation efficiency of PROTAC molecules.
Expanding the Target Range of PROTAC Technology: Traditional drug development mainly focuses on "druggable" target proteins with enzymatic activity or well-defined binding pockets. PROTAC technology, in conjunction with the use of target protein ligands, offers a potential solution for targeting "undruggable" target proteins. By designing and screening ligands that specifically bind to these "undruggable" target proteins, and linking them with E3 ligase ligands to form PROTAC molecules, it is theoretically possible to degrade almost any target protein. This expands the target range for drug development and provides new strategies and opportunities for treating various diseases.
Facilitating the Design and Optimization of PROTAC Molecules: Target protein ligands are a crucial component of PROTAC molecules. Researchers can select appropriate target protein ligands based on different target proteins and research needs, and modify or optimize their structures. By altering the structure and chemical properties of target protein ligands, it is possible to adjust the PROTAC molecule's binding ability, affinity, selectivity, and cell permeability, thereby designing more efficient and safer PROTAC molecules.
How Target Protein Ligands Affect Drug Selectivity in PROTAC Technology?
Specific Binding
The specificity of the target protein ligand's binding ability is a fundamental factor affecting drug selectivity. If the target protein ligand can specifically recognize and bind to the target protein, the PROTAC molecule will be able to precisely recruit the E3 ligase to the target protein, reducing interactions with non-target proteins. For example, in PROTAC development targeting the BET protein family, due to the high homology among the BET protein family members, traditional small molecule inhibitors struggle to achieve high selectivity for BET subtypes. By designing target protein ligands with high affinity for specific BET subtypes and linking them with E3 ligase ligands to form PROTAC molecules, the selectivity for the targeted BET subtype can be improved, thus enhancing the drug's selectivity.
Synergistic Effect
The synergistic effect between the target protein ligand and the E3 ligase ligand also affects drug selectivity. In PROTAC molecules, the target protein ligand and the E3 ligase ligand work together to form a stable ternary complex. The strength and specificity of this synergistic effect determine the selectivity of PROTAC molecules for the degradation of the target protein. If the binding of the target protein ligand to the target protein and the E3 ligase ligand to the E3 ligase can both occur efficiently and specifically, the formation of the ternary complex will be more stable and effective, improving the drug's selectivity for the target protein. For example, studies have shown that by optimizing the structure of target protein ligands and E3 ligase ligands, as well as their linkage, the synergistic effect of PROTAC molecules can be enhanced, thereby improving their selectivity for the degradation of specific proteins and reducing off-target effects.
Antigen-Antibody Reaction
The binding of the target protein ligand to the target protein is similar to the antigen-antibody reaction, and the specificity and affinity of this reaction are crucial for drug selectivity. High-affinity target protein ligands can bind more stably to the target protein, allowing PROTAC molecules to act longer within the cell, thereby improving the degradation efficiency and selectivity of the target protein. At the same time, the specificity of the antigen-antibody reaction ensures that PROTAC molecules degrade only the target protein without cross-reacting with other proteins that have similar structures or functions, thus reducing non-selective toxicity.
Impact on Ternary Complex Formation
The structure and properties of the target protein ligand affect the formation of the ternary complex. A suitable target protein ligand can promote the stable formation of the ternary complex, whereas a poorly structured ligand may cause the complex to be unstable, which would affect drug selectivity. For example, the size, shape, and charge distribution of the target protein ligand can influence its interaction with the target protein and the E3 ligase ligand, thereby affecting the formation and stability of the ternary complex. Only a stable ternary complex can effectively initiate the ubiquitination and degradation of the target protein and ensure that the process is highly selective.
How Target Protein Ligands Affect Non-Selective Toxicity of Drugs?
Specificity of the Target Protein Ligand
Impact: The specificity of the target protein ligand directly determines the accuracy with which PROTAC molecules bind to the target protein. If the target protein ligand is not highly specific, it may bind to non-target proteins with similar structures or homologous sequences, leading to the degradation of non-target proteins and causing non-selective toxicity.
Example: In PROTAC development targeting the BET protein family, if the target protein ligand cannot distinguish between different BET subtypes, it may degrade multiple subtypes simultaneously. As the BET protein family is involved in various physiological functions within cells, this non-selective degradation could disrupt normal cellular processes and result in toxicity.
Stability of the Ternary Complex
Impact: The stability of the binding between the target protein ligand and the target protein, as well as the stability of the binding between the E3 ligase ligand and the E3 enzyme, jointly affects the formation of the ternary complex. An unstable ternary complex may lead to inaccurate ubiquitination and degradation of the target protein, or off-target binding, thereby increasing the risk of non-selective toxicity.
Example: If the binding affinity of the target protein ligand to the target protein is weak and it dissociates easily in the complex cellular environment, PROTAC molecules might bind to other non-target proteins, forming incorrect binary or ternary complexes, leading to the degradation of non-target proteins.
Drug Concentration and Exposure Time
Impact: Drug concentration and exposure time affect the interaction between the target protein ligand and the target protein, as well as between the E3 ligase ligand and the E3 enzyme. Excessively high concentrations of PROTAC drugs or prolonged exposure times may lead to excessive degradation of the target protein, even affecting the normal function of non-target proteins, resulting in non-selective toxicity.
Example: Some studies have found that when the concentration of PROTAC drugs exceeds a certain threshold, a "hook effect" occurs, where the number of binary complexes formed with the target protein increases, thereby reducing the degradation efficiency of the target protein. Additionally, it may lead to non-specific interactions with other non-target proteins, causing increased cellular toxicity.
Drug Delivery and Distribution
Impact: The properties of the target protein ligand can affect the delivery and distribution of PROTAC drugs in the body. If the target protein ligand causes the PROTAC drug to accumulate in non-target tissues or cells, non-target proteins in these areas may be degraded, leading to non-selective toxicity.
Example: Some target protein ligands may have tissue tropism, leading to the accumulation of PROTAC drugs in specific non-target tissues. This can result in the degradation of non-target proteins in these tissues, causing cellular damage.
Selectivity of E3 Ligase
Impact: The expression of E3 ligases varies across different tissues and cells. The degradation selectivity of a PROTAC drug, formed by combining the target protein ligand with a specific E3 ligase ligand, can be influenced by the distribution of E3 ligases.
Example: If the chosen E3 ligase is highly expressed in many normal tissues, the PROTAC drug may cause the degradation of non-target proteins in these tissues, leading to non-selective toxicity.
Protein Turnover and Synthesis
Impact: The turnover rates and synthesis rates of different proteins vary. If the PROTAC-induced degradation of the target protein does not match the normal turnover and synthesis rates of that protein, it can lead to a disruption in the balance of proteins within the cell, resulting in non-selective toxicity.
Example: If the PROTAC drug degrades the target protein much faster than its synthesis rate, the target protein may remain at low levels within the cell for extended periods, disrupting normal cellular functions and potentially leading to cell death.
How to Design an Efficient Target Protein Ligand?
Target Selection and Ligand Binding Properties
Selecting the Right Target: Choose a target protein that has a clear biological function, is closely related to the disease, and possesses a structurally accessible binding pocket and active site.
High Affinity Binding: Use computer-aided drug design (CADD) tools, such as molecular docking, to predict and optimize the binding modes of the ligand to the target protein, thereby improving affinity. Additionally, based on structure-based drug design (SBDD), analyze the three-dimensional structure and active site of the target protein to design a ligand structure that precisely matches.
Specific Binding: Utilize DNA-encoded compound libraries (DEL) technology to screen for specific ligands that can rapidly find small molecule ligands specifically binding to the target protein without structural information. Furthermore, analyze the structural and sequence characteristics of the target protein to avoid cross-reactivity with structurally similar non-target proteins.
Consider the Synergy of Protein-Ligand Interactions
Stability of the Ternary Complex: The designed target protein ligand should work synergistically with the E3 ligase ligand to form a stable and cooperative ternary complex. The design of the linker is crucial for forming a stable ternary complex. Its length, flexibility, and chemical composition need to be precisely adjusted to fit the spatial steric and interaction requirements of the target protein and E3 ligase.
Optimizing Protein-Ligand Interactions: Use deep learning tools such as LigandMPNN to optimize sequence design tasks, improving the binding efficiency and specificity between the target protein and the ligand.
Enhancing Drug Stability in Vivo
Introducing Lipophilic Groups: Introduce appropriate lipophilic groups, such as alkyl chains or aromatic rings, into the ligand structure to enhance its stability in vivo.
Optimizing Chemical Structure: Incorporate electron-withdrawing groups, such as halogen atoms, into the ligand molecule to alter the molecular electron distribution and enhance stability. Use bioisosteric replacement principles to replace active groups with similar-sized and electronically similar groups to improve stability.
Considering the Pharmacokinetic Properties of the Drug
Improving Bioavailability: The molecular weight of the ligand should not be too large and should be kept within a certain range (typically below 500 Daltons) to enhance its ability to cross cell membranes. Increasing the lipophilicity of the ligand can also help improve bioavailability.
Extending Half-Life: Introduce groups with strong plasma protein binding capacity, such as aromatic rings or heterocycles, into the ligand structure to extend its half-life in vivo.
Other Design Strategies
Covalent Binding Strategy: Develop covalent target protein ligands to achieve irreversible binding to the target protein and improve degradation efficiency. However, it is important to select the appropriate target to avoid off-target effects.
Dual-Mechanism Degraders: Combine two degradation mechanisms in PROTAC molecules to improve degradation efficiency and selectivity.
Non-Specific Protein Targeting Approaches: For non-specific proteins, strategies like light-controlled linker-targeted release, constructing PAC molecules, or designing based on the specificity of E3 ligases can be used to improve targeting.















![N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-2-butenamide](https://resource.bocsci.com/structure/439081-18-2.gif)





