What is PROTAC Linker?
Within a PROTAC molecule the PROTAC linker acts as a chemical chain between the target protein ligand and the E3 ubiquitin ligase ligand. The PROTAC linker functions as an essential component since it determines crucial physicochemical properties like structural rigidity and flexibility while also influencing target protein degradation through ternary complex formation and cell permeability.
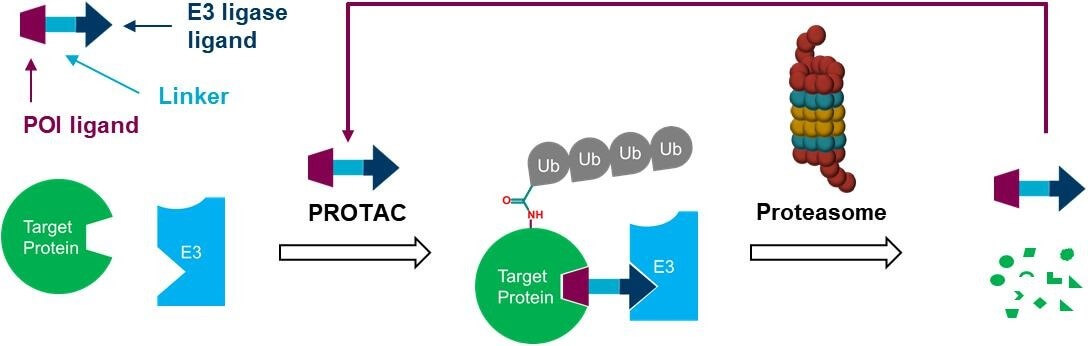
Functional Roles
Bridging Ligands: The linker acts as a bridge between the target protein ligand and the E3 ubiquitin ligase ligand, enabling the PROTAC molecule to simultaneously bind both the target protein and the E3 ligase. This facilitates the formation of a ternary complex, thereby triggering ubiquitination and subsequent degradation of the target protein.
Regulating Steric Hindrance: The length and flexibility of the linker must be appropriately balanced. A linker that is too short may hinder the formation of the ternary complex, while one that is too long could compromise the degradation activity. An optimal length and flexibility ensure effective proximity and stable complex formation between the target protein and E3 ligase.
Influencing Physicochemical Properties: The chemical composition of the linker affects the hydrophilicity or hydrophobicity of the PROTAC molecule, which in turn influences its pharmacokinetics and bioavailability in vivo.
Common Types
Alkyl Linkers: These have simple structures and provide stable connectivity with some degree of flexibility. They are widely used in many PROTAC molecules, especially those with 6–12 carbon atoms in length, which help accommodate different protein conformations.
Polyethylene Glycol (PEG) Linkers: Known for excellent water solubility, PEG linkers enhance the solubility and cell permeability of PROTAC molecules. Their length and flexibility are tunable, with PEG linkers commonly composed of 3 to 12 ethylene glycol units.
Rigid Linkers: Linkers containing aromatic rings or double bonds introduce structural rigidity by limiting the conformational flexibility of the PROTAC molecule. This can stabilize the ternary complex conformation and enhance degradation activity. Benzamide-based rigid linkers are a typical example.
Design Considerations
- Length: The linker should be optimized to ensure the ideal spatial distance between the target protein and the E3 ligase. This is typically within a range of 6 to 24 atoms, though the optimal length may vary depending on the specific target protein and E3 ligase pairing.
- Flexibility vs. Rigidity: Moderate flexibility allows the molecule to accommodate the spatial structural variations of proteins and improve complex formation. In contrast, suitable rigidity can help lock in an active conformation and enhance degradation activity.
- Chemical Properties: Factors such as hydrophilicity, hydrophobicity, and charge distribution must be considered. For instance, PEG enhances water solubility; hydrophobic linkers can affect membrane permeability; and charged groups can influence molecular interactions with proteins.
- Metabolic Stability: To prolong the in vivo half-life of PROTAC molecules, linkers should avoid structural motifs easily recognized and metabolized by enzymes. For example, long-chain alkyl linkers prone to oxidation should generally be avoided.
How Does the PROTAC Linker Affect Drug Stability?
Influence on Ternary Complex Stability
Length: The linker length must be optimal. A linker that is too short may introduce steric hindrance, preventing the target protein and E3 ligase ligand from coming into proximity, thereby reducing the probability of ternary complex formation. Conversely, an overly long linker may result in a loose complex with decreased stability. For example, studies have shown that shorter linkers like dBET57 are less effective in forming stable interactions with the E2/Ub catalytic site's lysine residues, which are essential for efficient degradation.
Flexibility: A linker with moderate flexibility can increase the likelihood that the E3-PROTAC-POI (protein of interest) complex adopts a favorable conformation for ubiquitination, thus improving degradation efficiency. However, excessive flexibility can hinder stable binding. Rigid linkers, on the other hand, can pre-organize the E3-PROTAC-POI complex into a catalytically favorable orientation, enhancing catalytic efficiency. For example, during the optimization of ARV-110, introducing a piperidine group into the linker increased its rigidity, leading to a more stable binding conformation between the AR ligand, thalidomide, and the E3 ligase, which improved ternary complex stability and enhanced AR degradation efficiency.
Influence on Physicochemical Properties
Hydrophilicity vs. Hydrophobicity: Highly lipophilic linkers, such as long alkyl chains, can reduce the hydrophilicity of the drug, negatively affecting its pharmacokinetics, including absorption, metabolic stability, and bioavailability. They may also increase the risk of hemolysis. On the other hand, overly hydrophilic linkers, such as excessively long PEG chains, can increase the polar surface area, reduce cell permeability, and hinder target protein degradation.
Chemical Stability: Some linker chemistries are more chemically stable and less prone to degradation reactions in vivo. For instance, linkers designed with azacyclic (nitrogen-containing) structures tend to have higher chemical stability, reducing the risk of PROTAC inactivation due to chemical degradation and thereby improving drug stability.
Influence on Metabolic Stability
Structural Stability: Structurally stable linkers, such as those based on cycloalkanes, can enhance the metabolic stability of PROTAC molecules, prolonging their activity in vivo. For example, in ARV-110, replacing the original flexible linker with a more rigid structure incorporating piperidine and piperazine significantly improved its metabolic stability.
Steric Effects: If the linker's attachment sites are strategically chosen to avoid steric hindrance that might interfere with ligand binding to the target protein, this can improve the in vivo stability and activity of the PROTAC. Additionally, well-designed attachment points can facilitate protein-protein interactions between the POI and E3 ligase, enhancing ternary complex stability and, in turn, the overall drug stability.
Influence of Special Types of Linkers
Photosensitive Linkers: For example, in pc-PROTAC, the PROTAC remains stable under protection from a photosensitive group. When exposed to light of a specific wavelength, the photosensitive group is removed, activating the PROTAC and triggering target protein degradation. This type of linker allows for spatiotemporal control of PROTACs, reducing toxicity to non-target tissues and, from another perspective, enhancing the stability and safety of the drug's use.
Self-Destructive Linkers: These linkers degrade after the PROTAC has exerted its effect, preventing long-term accumulation of PROTAC in the body. This reduces potential toxicity and side effects, enhancing the drug's safety and indirectly ensuring the overall stability of the drug.
How Does the Length of the PROTAC Linker Affect Drug Efficacy?
Influence on Ternary Complex Formation and Stability
Short Linkers: A linker that is too short may hinder the simultaneous binding of the target protein ligand and the E3 ligase ligand due to steric hindrance, thereby obstructing the formation of the ternary complex.
Long Linkers: A linker that is too long may increase the distance between the target protein and the E3 ligase, preventing the formation of a stable complex, and thus reducing the ubiquitination efficiency of the target protein.
Optimal Length Linkers: A linker of optimal length ensures that the target protein ligand and E3 ligase ligand bind within an appropriate spatial range, promoting ternary complex formation and enhancing stability. This, in turn, leads to more effective ubiquitination and degradation of the target protein. For example, in ER degradation experiments, a linker of 16 atoms in length (compound 13) exhibited the best performance in ER degradation.
Influence on Target Protein Degradation Efficiency
Optimal Length Varies by Target Protein and E3 Ligase Combination: For example, for a PROTAC targeting ERα, a PEG linker of 16 atoms in length exhibited stronger activity in ERα degradation compared to a 12-atom PEG linker. However, for PROTACs targeting TBK1 and VHL E3 ligases, degradation activity was not evident when the linker length was less than 12 atoms, but longer linkers significantly improved degradation potential.
There is an Optimal Length Range: Generally, linker lengths of 5-15 atoms are most common in PROTACs. For example, in the case of SOS1 degradation, among a series of PROTACs containing 3-9 methylene units, the PROTAC 8c (ZZ151) with a linker containing 5 methylene units showed the strongest activity, completely degrading SOS1. It also demonstrated excellent values for the half-maximal degradation concentration (DC50) and maximum degradation efficiency (Dmax).
Impact on Cell Permeability and Bioavailability
Long Linkers: A linker that is too long can increase the molecular weight and polar surface area of the PROTAC, reducing its cell permeability and impacting the drug's bioavailability.
Optimal Length Linkers: A linker of optimal length helps balance the hydrophilicity and lipophilicity of the PROTAC, allowing it to more effectively cross the cell membrane and exert its action within the cell.
Impact on Drug Metabolic Stability
Long Linkers: Linkers that are too long often have higher flexibility, which may make the PROTAC more susceptible to degradation by metabolic enzymes in the body, reducing the drug's metabolic stability.
Optimal Length Linkers: A linker of optimal length helps improve the metabolic stability of the PROTAC, extending its half-life in the body and thereby enhancing the drug's efficacy.
Potential Impact on Drug Specificity and Safety
Short or Long Linkers: A linker that is too short or too long may lead to non-specific binding of the PROTAC to off-target proteins, causing unwanted side effects and reducing the drug's specificity and safety.
Optimal Length Linkers: A linker of appropriate length helps improve the PROTAC's specificity in recognizing and binding to the target protein, reducing its impact on off-target proteins and thereby enhancing the drug's safety and tolerance.














![Tris[[2-(tert-butoxycarbonyl)ethoxy]methyl]methylamine](https://resource.bocsci.com/structure/175724-30-8.gif)






