AbTAC Degradation Technology Development
At BOC Sciences, we offer fully customized Antibody-based PROTAC (AbTAC) development services that harness the power of bispecific antibodies to achieve selective extracellular and membrane protein degradation.
Request a Consultation Explore ServicesCurrently, targeted protein degradation (TPD) drug discovery has been gaining increased attention the industry. As an emerging TPD technology, antibody-based PROTACs are developed that overcome the limitations of classical PROTACs that can only target intracellular proteins. As a leading provider of antibody engineering and targeted protein degradation solutions, we offer comprehensive Antibody-based PROTAC (AbTAC) custom services to support clients in designing, optimizing, and validating bispecific antibody degraders. AbTACs leverage antibody specificity to induce the ubiquitin-proteasome-mediated degradation of extracellular or membrane proteins by recruiting endogenous E3 ligases - offering a novel strategy for therapeutic intervention beyond traditional PROTACs.
What is AbTAC?
Antibody-based PROTAC (AbTAC) differs dramatically from classical small molecule PROTAC. In terms of molecular composition, AbTAC is a fully recombinant bispecific antibody, representing a new prototype within the field of PROTAC, whereas classical PROTAC is a three-part small molecule. In terms of degradation system, AbTAC targets membrane proteins and uses the lysosomal system to achieve degradation of the target protein. Rather than being a PROTAC derivative, AbTAC is more like a complementary approach to LYTAC in that both proteins are recruited for lysosomal degradation and no major cellular proteomic perturbations occur. Specifically, AbTAC is a bispecific immunoglobulin G (IgG) that recognizes two distinct epitopes. An arm of AbTAC recruits E3 ligase (e.g., transmembrane E3 ligases ring finger 43, RNF43) and the other arm targets cell surface protein (e.g., programmed death-ligand 1, PD-L1), inducing the formation of E3 ligase-AbTAC-protein complex and subsequent degradation. AbTAC has been reported to induce internalization and lysosomal degradation of PD-L1 by recruiting the RNF43. AbTAC mediates protein degradation by harnessing the endosomal-lysosomal pathway, but its mechanism of action remains elusive, and it is unknown whether the intracellular region of POI is ubiquitinated prior to endocytosis.
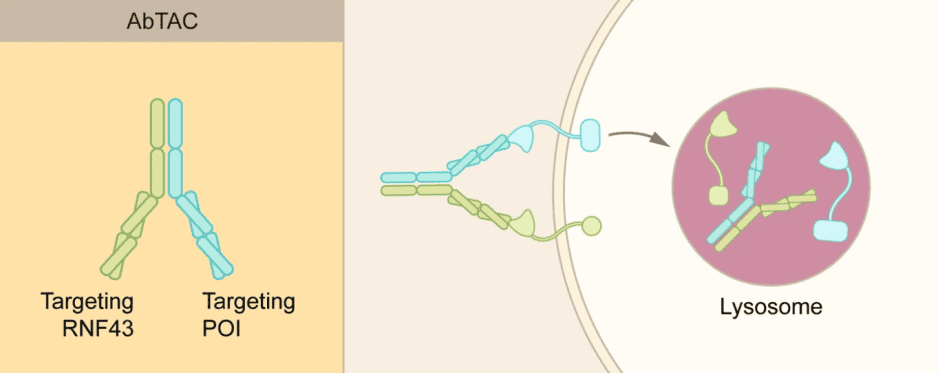 Fig 1. AbTAC (Zhao, 2022)
Fig 1. AbTAC (Zhao, 2022)
Services
Custom Antibody-based PROTAC (AbTAC) Development Services
Target Selection and Evaluation
Assessment of biological function and druggability of target proteins
Expression level analysis of membrane or extracellular proteins
Evaluation of co-localization potential with E3 ligases (e.g., RNF43, TRIM21)
Antibody Discovery and Optimization
Screening of high-affinity antibodies (monoclonal, humanized, bispecific) targeting specific proteins
Antibody engineering services, including Fc region modification and conjugation site optimization
Antibody production and purification using CHO or HEK293 expression systems
E3 Ligase Antibody Generation
Discovery of antibodies specifically recognizing E3 ligases (e.g., RNF43)
Design and development of bispecific antibodies: one arm binds the target protein, the other binds the E3 ligase
AbTAC Construction and Validation
Antibody-antibody fusion or chemical conjugation (e.g., Sortase A-mediated ligation, click chemistry)
Purification and structural confirmation of AbTAC molecules (SEC, SDS-PAGE, LC-MS)
Degradation activity analysis via Western blot, flow cytometry, and protein quantification assays
Cellular Assays and Mechanistic Studies
In vitro validation of AbTAC-mediated protein degradation and pathway modulation
Degradome profiling using proteomics to assess degradation specificity
Cytotoxicity and target selectivity evaluation in relevant cell lines
In Vivo Efficacy and PK/PD Analysis (Optional)
In vivo protein degradation analysis in mouse or non-human primate models
Biodistribution, half-life, and immunogenicity assessments
Optional Add-On Services
Antibody conjugation process development (ADC/AbTAC/antibody-enzyme conjugates)
Antibody stability testing (DSF, SEC-MALS)
Advantages
Why Choose Us for AbTAC Services?
01.Comprehensive One-Stop AbTAC Development Platform
We offer end-to-end AbTAC development services—from antibody discovery and engineering to AbTAC construction, functional validation, mechanistic studies, and in vivo evaluation. This integrated solution accelerates project timelines and minimizes the complexity and cost of outsourcing coordination.
02.Advanced Antibody Engineering and Discovery Capabilities
Our mature antibody platform features cutting-edge discovery technologies, including single B cell screening and phage display, combined with robust antibody engineering (e.g., Fc region modification, bispecific design, stability enhancement, and low-immunogenicity optimization). We support the generation of diverse AbTAC formats to meet various therapeutic needs.
03.Flexible and Diverse Conjugation Strategies
We offer a broad portfolio of site-specific conjugation and fusion methods, such as Sortase A-mediated ligation, click chemistry, and linker screening and optimization. These strategies ensure stable and functional AbTAC molecule assembly.
04.High-Throughput Protein Degradation Validation
Our robust in vitro and cellular validation systems include Western blotting, flow cytometry, and quantitative proteomics (e.g., TMT-labeling) to quickly confirm AbTAC-induced degradation efficiency and target specificity.
05.Expert Target Evaluation and Mechanism-of-Action Studies
Our interdisciplinary scientific team provides comprehensive support for target degradability assessment and E3 ligase compatibility. We help elucidate AbTAC mechanisms of action and evaluate degradation pathway dependency (E3 ligase, lysosomal, or proteasomal pathways).
06.Extensive E3 Ligase Antibody Library and Screening Capabilities
We maintain a diverse library of high-affinity antibodies and ligands targeting E3 ligases such as RNF43 and TRIM21. These can be flexibly paired with target-binding antibodies to enable versatile and customizable AbTAC strategies.
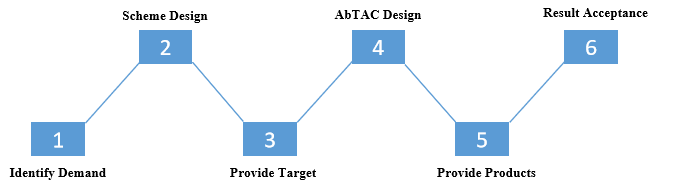
Workflow
Workflow of PROTAC Design Services
Platform
Technological Platforms
Our AbTAC service offering is built upon an integrated suite of advanced technology platforms that collectively enable the efficient discovery, engineering, construction, and validation of high-performance AbTAC molecules.
Applicaiton
Application Areas
Immuno-oncology Applications
Facilitate degradation of immune checkpoint proteins to enhance anti-tumor immune responses.
Neuroscience Research
Study and manipulate cell-surface receptors involved in neurodegenerative and psychiatric disorders.
Autoimmune Disease Intervention
Downregulate pathogenic surface proteins to modulate overactive immune responses.
Drug Resistance Mechanism Studies
Investigate resistance-associated surface proteins and their functional elimination via AbTAC.
Preclinical Antibody Degrader Development
Accelerate the generation of first-in-class antibody degraders for novel biologics pipelines.
E3 Ligase Biology Research
Explore new E3 ligase biology and their therapeutic potential in extracellular protein degradation.
Discover Additional Solutions for Your Research

- Precise Screening
- Cellular Phenotypic Assays
- Targeted Degradation Profiling

- Pharmacodynamics Monitoring
- Tissue-Specific Degradation
- Long-Term Toxicity Studies

- Ligase Engineering
- Ligase-Targeted Synergy
- Modular Ligase Systems

- High-Yield Expression
- Protein Engineering
- Biochemical Characterization

- Lysosomal Targeting
- Novel Degradation Pathways
- Selective Autophagy Activation

- Enhanced Ubiquitination
- Improved Targeting Effect
- Degradation Rate Modulation

- Personalized Drug Design
- Multitargeted Drug Design
- Disease-Driven Optimization

- Targeted Custom Design
- High Selectivity and Efficiency
- Enhanced Affinity
Further Reading on Related Topics
FAQ
Frequently Asked Questions (FAQs)
1. What types of targets are suitable for AbTACs?
AbTACs are particularly suited for degrading membrane proteins and extracellular targets such as GPCRs, RTKs, and immune checkpoint molecules - which are typically inaccessible to small-molecule PROTACs.
2. Which E3 ligases can be used in AbTAC design?
Common E3 ligases employed include RNF43, TRIM21, and MARCH8, selected based on cellular localization and expression. We also support custom screening for novel or cell-type specific E3 ligases.
3. Can you help design bispecific antibodies for AbTAC?
Yes. Our team provides end-to-end design, including epitope mapping, affinity tuning, and bispecific antibody formatting (e.g., scFv-Fc, IgG-like). We also offer linker engineering and conjugation solutions.
4. Do you offer in vitro or in vivo functional testing?
Absolutely. We provide both in vitro degradation assays and in vivo PK/PD evaluation, using reporter cell lines, flow cytometry, western blotting, and humanized mouse models for AbTAC efficacy testing.

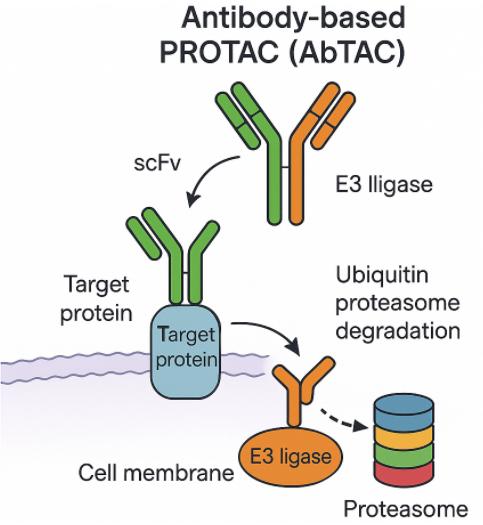
 Fig 1. AbTAC (Zhao, 2022)
Fig 1. AbTAC (Zhao, 2022)