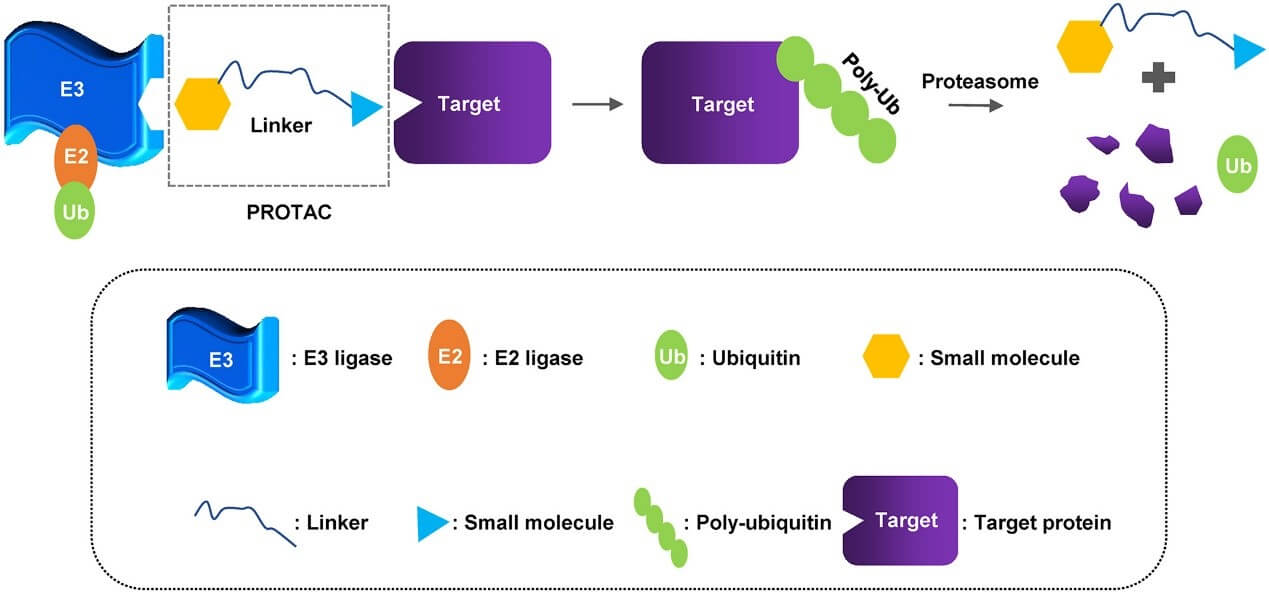
What is PROTAC?
PROTAC® is the abbreviation for proteolysis targeting chimera, which is a small bifunctional molecule. One end of the molecule is a ligand that binds the target protein and the other end is a ligand that binds E3 ubiquitin ligase. The two ends are connected by a chain. In vivo, the target protein and E3 ubiquitin ligase can be drawn closer, so that the target protein is labeled with ubiquitin and then degraded by the ubiquitin-proteasome pathway. This is a brand-new drug design strategy. By designing such triplet small molecule drugs, theoretically, any over-expressed and mutated pathogenic protein can be eliminated for the purpose of treating the disease.
Development History
- Early Stage: In 2001, researchers proposed the concept of small molecule-induced protein degradation and designed a chimeric molecule composed of an estrogen receptor ligand, an IκBα peptide segment, and an E3 ubiquitin ligase ligand. This represented the early design concept of PROTACs, but the molecule failed to deliver the expected results due to poor cell membrane permeability.
- Initial Development Stage: In 2008, researchers enhanced the inhibitory activity against NF-κB by modifying the IκBα-derived chimeric molecule. In 2012, the first cell-permeable PROTAC molecule was reported, composed of a HIF-1α-derived peptide and a VHL-derived peptide connected via a disulfide bond, which effectively degraded the androgen receptor. In 2013, it was discovered that PROTACs based on VHL-derived peptides could degrade various estrogen receptors.
- Rapid Development Stage: Starting in 2015, the discovery that CRBN could serve as an E3 ligase in PROTAC design significantly advanced the field. Since then, various CRBN-based PROTAC molecules have been developed to degrade multiple pathogenic proteins, such as androgen receptors, estrogen receptors, and BTK.
Structural Components
Target Protein Ligand: This is the domain within the PROTAC molecule responsible for recognizing and binding to the target protein, which determines the specificity of the PROTAC. For example, Panobinostat is a PROTAC molecule targeting HDAC6, and its HDAC6 ligand component can precisely bind to the HDAC6 protein.
E3 Ubiquitin Ligase Ligand: E3 ubiquitin ligases play a key role in the ubiquitin-proteasome system. The PROTAC molecule brings the target protein close to the E3 ubiquitin ligase via this ligand, allowing the target protein to be tagged with ubiquitin. Commonly used E3 ligase ligands include VHL and CRBN. For instance, ARV-110 uses a CRBN ligand to facilitate the interaction between the androgen receptor and the CRBN-E3 ubiquitin ligase.
Linker: The main function of the linker is to connect the target protein ligand and the E3 ubiquitin ligase ligand. Its length and chemical properties can affect the activity, selectivity, and pharmacokinetics of the PROTAC molecule. In some PROTACs, a longer linker may help enhance degradation activity.
Mechanism of Action
PROTAC technology primarily utilizes the intracellular ubiquitin-proteasome system to achieve degradation of target proteins. Once the PROTAC molecule enters the cell, its target protein ligand binds to the target protein, while the E3 ubiquitin ligase ligand binds to the E3 ligase, forming a ternary complex. The formation of this complex enables the E3 ligase to specifically recognize and attach ubiquitin chains to the target protein. The ubiquitinated target protein is then recognized and degraded by the proteasome within the cell.
Advantages of PROTAC
- Targeted Degradation: PROTACs can directly induce the degradation of target proteins, thereby more thoroughly eliminating their function. Compared to traditional inhibitors, PROTACs offer more durable and complete target engagement.
- Overcoming Drug Resistance: For proteins that have developed resistance due to genetic mutations, PROTACs can still exert therapeutic effects by inducing their degradation, thus overcoming resistance issues associated with small-molecule inhibitors.
- Expanded Target Scope: PROTACs do not require specific structural features in the target protein, meaning they can, in theory, target nearly all intracellular and transmembrane proteins. This includes proteins previously considered "undruggable," such as non-enzymatic proteins and certain transcription factors, offering new therapeutic opportunities for difficult-to-treat diseases.
- Dosage Advantage: Since PROTAC molecules can be released and reused after facilitating protein degradation, they may achieve therapeutic effects at lower doses compared to traditional small-molecule inhibitors. This can potentially reduce drug-related toxicities and side effects.
Limitations of PROTAC
- Large Molecular Weight: Due to the presence of two ligands and a linker, PROTACs tend to have high molecular weights, which can limit cell membrane permeability and bioavailability, thereby constraining their in vivo drug development potential. Researchers are actively working on structural optimization to improve their cell permeability and pharmacokinetic properties.
- Off-Target Effects: While PROTACs are designed for high specificity, their mechanism—which involves recruiting E3 ubiquitin ligases and other intracellular components—may inadvertently lead to the degradation of non-target proteins, causing undesirable side effects. Therefore, thorough assessment and control of off-target effects are crucial during design and development.
- Stability Issues: The in vivo stability of PROTACs remains a concern. Their complex structures may be prone to degradation or metabolic inactivation in the biological environment, affecting their therapeutic efficacy. Chemical modifications and structural optimizations are being pursued to enhance their stability.
- Drug Resistance: Although PROTACs can, to some extent, overcome the resistance seen with traditional small-molecule inhibitors, long-term use may still lead to resistance via new mechanisms—such as reduced cellular uptake, increased metabolism, or accelerated excretion of PROTACs. Further research is needed to understand and overcome these emerging resistance mechanisms.
Applications of PROTAC
Cancer Therapy: PROTAC technology has made significant strides in cancer treatment. For example, ARV-110 and ARV-471, which target prostate and breast cancer respectively, have entered Phase II clinical trials. Additionally, various PROTACs targeting different proteins—such as HCK-PROTAC, CDK9 PROTAC, FGFR PROTAC, BET PROTAC, and FLT3 PROTAC—have demonstrated promising anti-tumor activity in preclinical studies.
Neurodegenerative Disease: In the field of neurodegeneration, PROTACs and other targeted protein degradation technologies are viewed as potential therapeutic approaches for diseases such as Alzheimer's disease, Parkinson's disease, and ALS. These strategies mainly focus on degrading pathogenic proteins such as tau, α-synuclein, and TDP-43.
Other Disease: Beyond oncology and neurodegeneration, PROTACs also show promise in treating inflammatory diseases and viral infections. For instance, PROTACs targeting IRAK4 hold potential in inflammatory disease treatment. Additionally, research is exploring antiviral applications, such as HB-PROTACs targeting HBx for the treatment of hepatitis B virus-related conditions.
The Role of CRBN in PROTAC Technology
CRBN plays a critical role in PROTAC (Proteolysis Targeting Chimera) technology. Its specific functions include:
Acting as the Substrate Recognition Receptor of an E3 Ubiquitin Ligase
CRBN is the substrate recognition receptor of the CRL4CRBN (Cullin 4-RING E3 ligase) complex, which can specifically recognize and bind to target substrate proteins. In PROTAC technology, a CRBN ligand is used to recruit CRBN, bringing it into close spatial proximity with the target protein, thereby initiating the ubiquitination and subsequent degradation of the target protein.
Enhancing PROTAC Function through Binding with IMiDs
CRBN is the direct target of immunomodulatory imide drugs (IMiDs), such as thalidomide. When IMiDs bind to CRBN, they alter its structure and function, enabling it to recruit specific substrate proteins for ubiquitination and degradation. This binding property forms the basis for designing CRBN-based PROTACs, allowing them to leverage the characteristics of IMiDs to enhance the degradation of target proteins.
Broad Expression and High Degradation Efficiency
CRBN is widely expressed in various cell types and tissues, which broadens the potential applications of CRBN-based PROTACs. Studies have shown that using CRBN as the E3 ubiquitin ligase in PROTACs results in efficient degradation of target proteins both in vitro and in vivo, effectively eliminating proteins associated with various diseases.
Favorable Drug-like Properties
Compared to some other E3 ubiquitin ligases, CRBN ligands offer better drug-like characteristics, such as lower molecular weight, better lipophilicity, and higher bioavailability. These features make CRBN-based PROTACs more likely to achieve good oral bioavailability and tissue permeability, thereby enhancing their therapeutic potential in vivo.
Enabling Greater Design Flexibility and Optimization
Due to differences in substrate recognition and degradation mechanisms between CRBN and other E3 ubiquitin ligases, CRBN-based PROTACs can complement other types of PROTACs in both design and application. Additionally, researchers can further improve the stability and activity of PROTACs through structural optimization of CRBN ligands, offering more possibilities for future PROTAC development.
How Do PROTAC Molecules Enter Cells?
The main mechanisms by which PROTAC molecules enter cells include:
Passive Diffusion
For PROTAC molecules with relatively small molecular weights and good lipophilicity, passive diffusion may allow them to cross the lipid bilayer of the cell membrane directly. However, since most PROTAC molecules are large and structurally complex, passive diffusion alone is often inefficient for effective cellular entry.
Endocytosis
Receptor-mediated endocytosis is one of the primary pathways through which PROTAC molecules enter cells. On April 17, 2025, researchers from the University of Texas and other institutions published a study in Cell, revealing that CD36 receptor-mediated endocytic cascades are a major mechanism for the transmembrane uptake of macromolecular and polar drugs. They proposed an "endocytic pharmacochemistry" strategy, whereby drug molecules are designed to more effectively bind CD36 receptors, thereby overcoming the size limitations imposed by the cell membrane and significantly enhancing intracellular delivery of large-molecule drugs.
Based on this strategy, the research team developed CD36-enhanced PROTACs, which demonstrated improved cellular uptake, higher protein degradation efficiency, and stronger anti-tumor activity. When a PROTAC molecule binds to the CD36 receptor on the cell surface, it triggers membrane curvature that leads to the formation of clathrin-coated pits or membrane invaginations. This process culminates in the formation of endolysosomes, which internalize the PROTAC. The contents of the endolysosome are then released inside the cell, allowing the PROTAC to exert its biological effects.
Prodrug Strategy
Chemical modification can be used to convert a PROTAC molecule into a prodrug by introducing removable groups to improve its biopharmaceutical or pharmacokinetic properties and enhance cellular uptake. For example, introducing lipophilic groups to mask key polar regions of the PROTAC via cleavable ester bonds can temporarily reduce the molecule's polarity, facilitating membrane permeability. Once inside the cell, these protective groups can be enzymatically or chemically cleaved in the intracellular environment to restore PROTAC activity.
Nanocarrier Delivery Systems
Rationally designed nanocarriers can help overcome the unfavorable physicochemical properties of PROTAC molecules, enabling tumor-specific accumulation, enhanced tissue penetration, and efficient cellular uptake. For instance, encapsulating PROTACs in nanoparticles allows for passive accumulation in tumor tissues via the enhanced permeability and retention (EPR) effect. Ligand modification strategies can further enhance the interaction between the nanoparticles and tumor cells, promoting endocytosis and ultimately delivering the PROTACs into the intracellular environment.

![(2S,4R)-N-((S)-2-(tert-Butyl)-17-((S)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)-4,16-dioxo-6,9,12-trioxa-3,15-diazaheptadecan-1-oyl)-4-hydroxy-1-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide](https://resource.bocsci.com/structure/1797406-69-9.gif)




















