In the past few years, there has been a great interest in a class of promising drugs, which do not work by inhibiting the molecular target as most traditional drugs do, but use the cell recovery system to destroy the target. However, it is difficult to find and design such unusual compound drugs, which are collectively called the molecular glue degraders.
Now, a new molecular glue-degrading agent called CR8 was discovered by scientists at the Broad Institute in the United States and the Friedrich-Michel Biomedical Institute in Basselle, Switzerland. By dissecting the details of the molecular mechanisms of CR8, these researchers show the possibility of building more of these unique compounds as potential treatments for various diseases. The results of the study were recently published in Nature, entitled "The CDK inhibitor CR8 acts as a molecular glue degrader that depletes cyclin K".
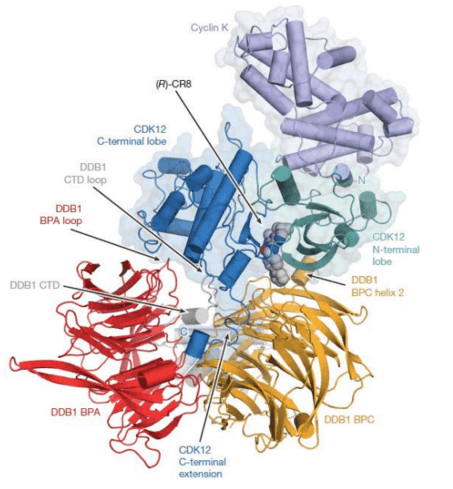
“By attaching a specific chemical group, it is possible to convert conventional kinase inhibitors into molecular glue degradants, which provides the potential to build molecular glue degradants for a wider range of therapeutic targets than we initially expected.” wrote Benjamin Ebert, co-author of the article and a cancer researcher at the Broad Institute.
Throw away the lock-and-key theory
Most drugs use lock-and-key mechanisms to target proteins (usually enzymes), blocking their activity by binding directly to unique grooves on the target proteins. However, many other types of proteins, such as transcription factors, lack such binding sites, hampering efforts to design drugs for these traditionally "undruggable" targets.
About six years ago, Ebert and his colleagues revealed that a well-known multiple myeloma drug called lenalidomide works as a molecular glue-degrading agent. Instead of directly binding to the target, it works by recruiting a molecular machine to label the target protein in the cell so that it can be subsequently destroyed. The molecular machine, called E3 ubiquitin ligase, attaches a small protein called ubiquitin to an ill-fated target, which is then degraded by the cell recovery system.
To identify more molecular glue degradants, the Ebert team, led by co-lead author Mikolaj Slabicki, a postdoctoral fellow at the Broad Institute, studied data on more than 4500 drugs and compounds from the Broad Institute Drug Reuse Center, which has collected the data on compounds that had been proven to be safe for humans, especially many compounds that have been approved by the U.S. Food and Drug Administration (FDA). They combed through the public data to determine the priority of drugs that kill cancer cells with higher levels of E3 ubiquitin ligase.
"We have been brainstorming in the lab, trying to find more molecular glue degradants. We are very lucky to have such a large and powerful data set. we would not have made such a discovery without this data set generated by the Broad Institute Cancer Project. " said Slabicki.
Ways to build more molecular glue degradants
CR8 is a compound originally designed to inhibit enzymes called cyclin-dependent kinase (CDK), which plays an important role in controlling cell growth. Using their bioinformatics methods, the researchers found that the cytotoxicity of CR8 is related to the level of a component called DDB1 in the E3 ubiquitin ligase complex.
The researchers found that CR8 kills cancer cells by inducing the degradation of a protein called cyclin K, which is the binding companion of some CDKs, especially CDK12. CR8 works like molecular glue. It binds to CDK12-cyclin K and recruits DDB1, and then recruits the rest of the E3 ubiquitin ligase complex to label cyclin K for subsequent degradation.
Researchers from the Bassel Friedrich-Michel Institute for Biomedical Research including Nicolas Thom, co-author of the paper, and co-lead authors Zuzanna Kozicka and Georg Petzold analyzed the crystal structure of the key components of the CR8-induced protein complex, revealing new molecular details of the interaction between all glued parts.
The group studied the activity of a drug with a structure similar to CR8 and found that it does not cause cyclin K degradation. The only structural difference between the two compounds is the protruding of a chemical group called pyridyl substituent. They concluded that the chemical group is enough to make CR8 act like a molecular glue degrader. This finding suggests that the extroverted parts of inhibitors can be chemically modified to be turned into molecular glue degradants targeting specific protein targets.
“Our findings suggest that we may be able to design these compounds ourselves, and there may also be many other molecular glue degradants, but they have not been discovered because the stability of their targets has not been studied. This is really exciting,” said Ebertd.
References
- Karali, C. S., & Mead, A. (2020). The Sticky Wicket of Undruggable Mutations: Can Targeted Molecular Degradation Help?. The Hematologist, 17(5).
- Słabicki, M., Kozicka, Z., Petzold, G., Li, Y. D., Manojkumar, M., Bunker, R. D., ... & Sperling, A. S. (2020). The CDK inhibitor CR8 acts as a molecular glue degrader that depletes cyclin K. Nature, 1-5.


