Substituting aliphatic alkyl tethers with piperazine or triazole units transform the linker from a passive strap to a pH-responsive, hydrogen bond-capable unit, offering additional handles to subtly modulate ternary complex geometry and whole-molecule physiochemistry. Piperazine provides a semi-rigid, basic loop, capable of being protonated in the endosome, to enhance solubility and promote membrane escape without the floppy entropy of PEG chains, while 1,2,3-triazole generated from click chemistry contributes a planar, metabolically stable ring capable of engaging in π-stacking or water-bridged contacts at the target–ligase interface. Proof-of-concept examples incorporating these motifs are already showing extended intracellular retention and enhanced oral exposure relative to their fully aliphatic analogs, hinting that functional heterocycles will become standard motifs in next-generation degrader design.
Introduction to Functional Linkers
Functional linkers intentionally incorporate chemical handles (ionizable nitrogens, hydrogen-bond acceptors/donors, or photo-switchable azo groups) within the spacer that connects the warhead and the E3-recruiting ligand. The objective is to transcend the historical conception of the linker as a passive tether solely responsible for imposing distance, instead endowing the spacer itself with a defined binding energy, the ability to modulate pH-dependent trafficking, or even to permit optical control of degradation. Piperazine and triazole have so far been the most commonly validated motifs because they are synthetically accessible, tolerant of diverse substitution patterns, and have well-characterized physicochemical signatures with known or predicted in silico properties.
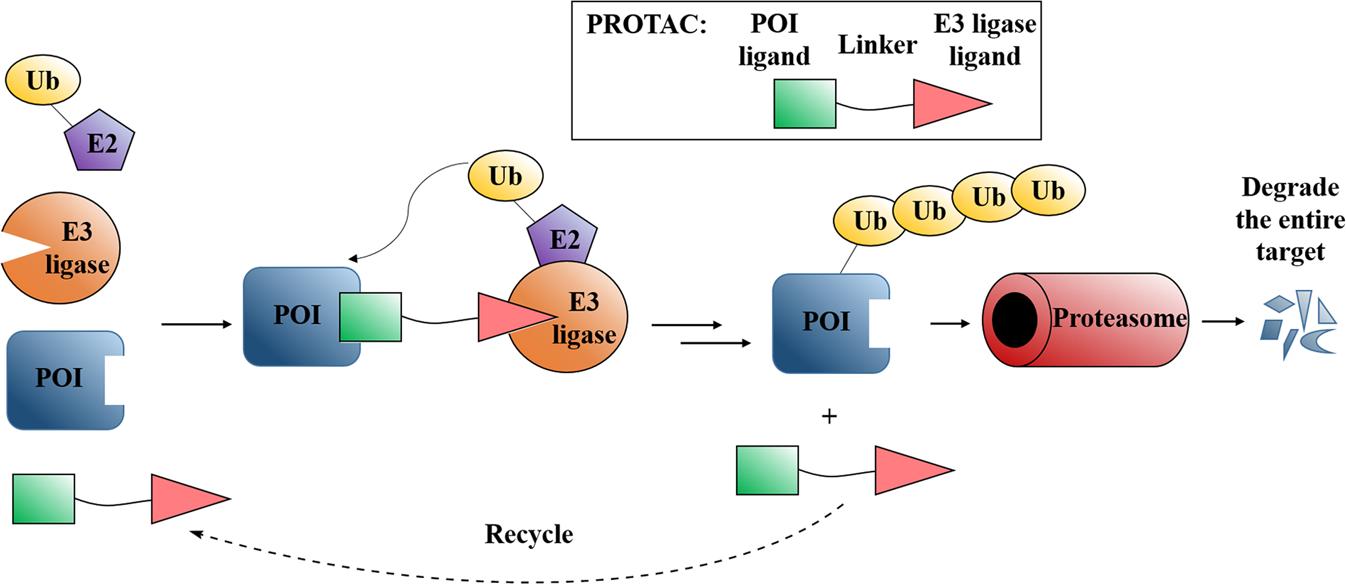 Fig. 1 Mode of action of PROTACs.1,5
Fig. 1 Mode of action of PROTACs.1,5
Why Beyond Simple Spacers?
Classic alkyl or PEG chains address the problem at the expense of conformational entropy and metabolic liability. The interlinker bonds are free to rotate, such that only a small fraction of the PROTAC population can achieve the productive pose that positions both binding moieties in the proper orientation at the same time, so larger doses are needed to promote ternary complex assembly and, by extension, the risks of off-target engagement and oxidative cleavage. Flexible linkers also afford no electronic complementarity to the protein surface; they are threaded through grooves without directional contacts and so residence time is at the mercy of the weak binary affinities of the terminal ligands. Piperazine, by contrast, inserts a semi-rigid six-membered ring containing a basic nitrogen whose protonation state is sensitive to the acidic milieu of the early endosome, promoting membrane release while remaining largely neutral at the cytosolic pH. Triazole, although formally aromatic, sports three electronegative nitrogens that endow the ring with a quadrupole moment that is complementary to edge-to-face stacking interactions against phenylalanine or tyrosine sidechains often encountered at the edge of the ternary interface. The following non-covalent extras hold the nascent complex together for a few extra milliseconds, time that the ubiquitin machinery capitalizes on to finish the transfer of poly-ubiquitin. In addition, both motifs are constructed from modular, high-yielding reactions (reductive amination for piperazine, copper-catalysed azide–alkyne cycloaddition for triazole) so that chemists can iterate over regioisomers and substitution patterns overnight rather than undertaking multi-step sequences. The strategic payoff is to transform what was once an entropic liability into an enthalpic and physiochemical asset without inflating the molecular weight or synthetic complexity.
The Role of Functional Linkers in PROTACs
In the cell, the linker is the only part of the PROTAC that is simultaneously solvent-accessible, adjacent to the protein–protein interface, and visible to metabolic enzymes; thus functionalizing this region provides a unique handle for global optimization. Piperazine can be mono-alkylated to form an asymmetric handle that vector the exit trajectory of the warhead, while leaving the distal nitrogen available for protonation-driven membrane translocation. Because the ring is semi-rigid, it also restricts the rotational freedom of adjacent segments, reducing the entropic penalty of ternary complex assembly compared with a PEG chain of similar length. Triazole makes a different contribution: its planar structure and polarized nitrogens are capable of accepting hydrogen bonds from backbone amides or structured water molecules, thus stapling the POI and E3 ligase in a catalytically competent orientation. Crystallographic snapshots of triazole-containing degraders display short water-bridged networks that span the triazole N-2, a histidine on the target, and a tyrosine on the ligase; these contacts are not observed in purely aliphatic analogues. Functionality also impacts PK: the basicity of piperazine increases polar surface area enough to enhance dissolution rate without provoking P-glycoprotein-mediated efflux, while the metabolic stability of the triazole ring protects the linker from cytochrome P450 attack, extending the time of intracellular exposure. Critically, both motifs are orthogonal to the binding chemistries of the warhead and the E3 ligand, and so their electronic effects can be overlaid on top of existing ligands without having to re-optimize the whole molecule. These factors in combination position functional linkers as strategic control elements that simultaneously tune binding cooperativity, metabolic lifetime, and physicochemical properties, transforming the formerly passive spacer into a pharmacologically active component of the PROTAC.
Piperazine Linkers
Positioned between the flexibility of alkyl chains and the rigidity of aromatic rods, piperazine linkers combine a saturated, conformationally constrained, heterocyclic core that can be protonated in acidic organelles. By sequestering a basic nitrogen in a six-membered ring, the linker acquires a modifiable ionization handle that can elevate aqueous solubility without the entropic cost of long PEG chains, while the semi-rigid backbone pre-organizes the two ligands into an active ternary conformation. Recent structure–activity work has shown that small changes in attachment angle or N-substitution pattern can have as dramatic an effect on cell potency as changing the warhead itself, revealing that piperazine is not just a solubilizing appendage but an active structural element.
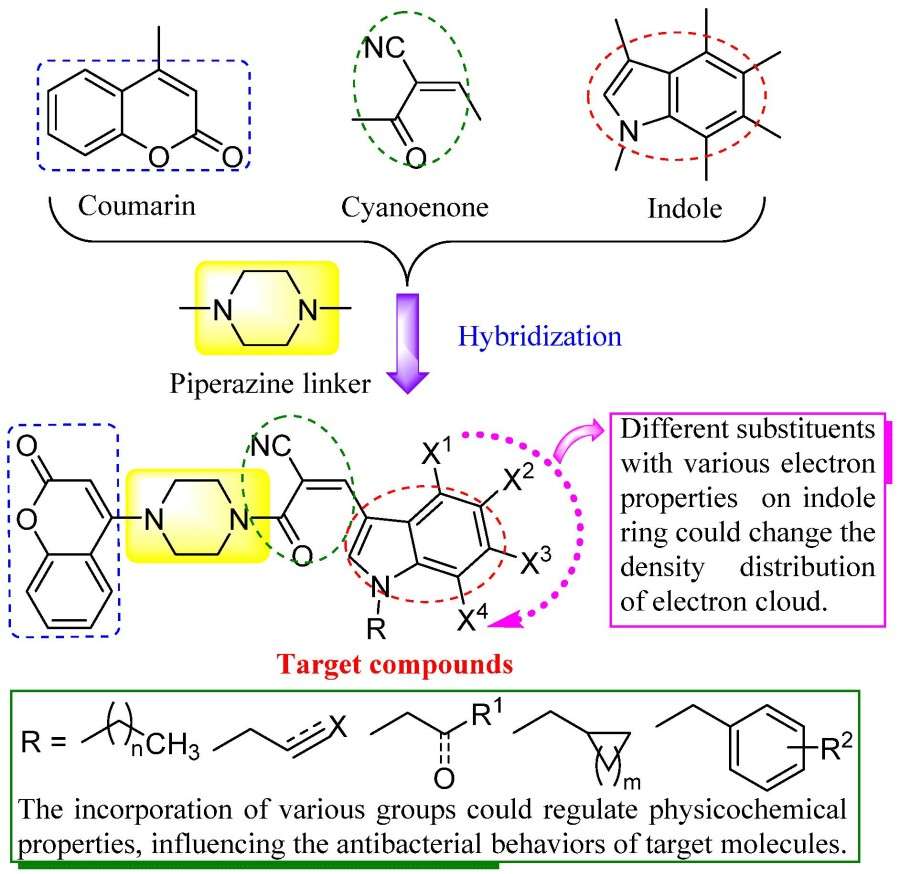 Fig. 2 Design of piperazine hybridized coumarin indolylcyanoenones as new structural antibacterial agents.2,5
Fig. 2 Design of piperazine hybridized coumarin indolylcyanoenones as new structural antibacterial agents.2,5
Solubility and Protonation Control
The solubility boon of piperazine, however, is not a free gift. It is a compromise between basicity and micro-environment. The ring nitrogens can be protonated, but the degree to which this occurs is very sensitive to the electron-withdrawing nature of the nearby linker groups. An amide or ester carbonyl γ or closer will decrease the pKa through inductive effects, even quenching protonation at physiological pH to a negligible level. On the other hand, the insertion of a polymethylene or ether spacer removes the nitrogen from the deshielding environment, giving back a nonzero basicity and allowing a partial cationic population to pull the whole package into the water phase. The result is an onboard solubilizer that can be turned on as necessary: in the mildly acidic early endosome the equilibrium shifts toward the charged form, preventing aggregation and avoiding precipitation that can often obscure the true DC50. At cytosolic pH the same molecule is largely uncharged, retaining enough lipophilicity to cross membranes and avoiding the renal rapid-clearance pitfall into which permanently charged motifs fall. Fine-tuning can be accomplished without reengineering the entire PROTAC; a simple amide/reverse amide swap or an extension of an alkyl tether by one unit can shift the protonation window by nearly an order of magnitude, giving medicinal chemists an intuitive knob to turn rather than a simple on/off switch. Since the ring is symmetrical, these modifications can be made at either nitrogen, permitting late-stage diversification while maintaining the same central axis, a freedom that few other basic backbones allow.
Structural Advantages and Applications
In addition to providing an ionizable handle, the piperazine ring enforces a conformational grammar which is hard to mimic with open-chain diamines. The chair conformation locks the two exit vectors at a fixed angle, thus lowering the rotational entropy that needs to be paid upon ternary complex formation; in practical terms, this translates to faster association kinetics and lower dissociation rates even without highly affinity warheads. The ring also serves as a mechanical fuse: in reductive conditions that can be encountered in some tumor microenvironments the N–N (or N–C) bond can be cleaved tracelessly, providing an in-built safety switch to limit off-target engagement after payload delivery. Architecturally, piperazine is compatible with orthogonal protection: one nitrogen can be Boc-masked while the other is alkylated, allowing sequential conjugation of ligase ligand and target binder without cross-reactivity. This feature has been used to build branched degraders where a single E3 recruiter is flanked by two different warheads, doubling the degradation footprint while keeping the molecular weight in the "beyond rule of five but still deliverable" corridor. Finally, the ring's symmetry eases regulatory characterization: only one set of 13C-NMR signals need to be assigned, and oxidative impurities—should they arise—are usually limited to the monomeric N-oxide, a species that is both easy to detect and straightforward to purge.
Triazole Linkers
The 1,2,3-triazole ring installed by azide–alkyne ligation is no longer considered an inert coupling scar; it has evolved into a conformational lock that can be dialed into any PROTAC blueprint without interfering with the warhead or the E3 recruiter. This functionality owes to an uncommon mix of chemical equanimity (tolerance of acids, bases, light and reactive oxygen species) and structural decisiveness: the planar five-membered ring fixes substituents at a 120° vector while providing a dipole moment that can hydrogen-bond to interfacial waters. The consequence is that the same reaction that ligates the linker also programs its downstream behavior, collapsing synthetic and functional design into a single operation.
Click Chemistry Reliability
The widespread use of copper(I)-catalyzed cycloaddition can be attributed to an exceptionally broad kinetic window: the rate is almost independent of the alkyne electronic character, and the azide can be protected inside a sterically demanding heterocycle or attached to a PEG terminus. This flexibility results in robustness on the bench. Byproducts are almost exclusively the 1,5-regioisomer or starting material, both chromatographically separable and trivial to remove. Water is not merely tolerated but actually accelerates the reaction through pre-organization of the azide by hydrogen bonding, thus negating the need for the often reaction-incompatible rigor of drying that disqualifies other cross-couplings for bioconjugation. Oxygen exclusion is relaxed rather than rigorous; the catalyst is trapped and stabilized by in-situ formed triazolide ligands, so a septum-flushed vial is good enough. Scale-up is linear: the same stoichiometry that produces milligram quantities in an Eppendorf tube produces gram quantities in a round-bottom flask without the need for re-optimization, because heat dissipation, not kinetic efficiency, is rate-limiting. Most importantly, the reaction is information-rich. The appearance of a single, well-resolved triazole proton in the NMR spectrum is an internal reporter: if the expected signal is absent or broadened, the culprit is protecting-group integrity or solubility, not the ligation itself. This self-diagnosing feature shortens the troubleshooting cycle and allows non-specialists to use triazole linkers in multi-step sequences that terminate in live-cell assays.
Rigidity and Orientation Benefits
The once-bound triazole acts as a molecular spline, inhibiting the linker from folding into a random coil. The planar heteroaromatic core imposes a 5-Å contour length that is reproducible in both force-field simulations and cryo-EM maps, so distance estimates on paper can be translated into fixed separations within the ternary complex. The ring also generates a permanent dipole whose negative pole is localized on N-3; this vector can accept backbone amide H-bonds that line the E3–target interface, effectively stapling the degrader in place for the microseconds required for ubiquitin transfer. In contrast to amide bonds, the triazole cannot undergo cis/trans isomerization, avoiding a source of conformational heterogeneity that can broaden thermal melting curves of PROTAC-bound complexes. Photostability is an added dividend: the conjugated π-system absorbs only in the far-UV, so routine microscopy or flow-cytometry illumination does not cause photobleaching or cross-linking artefacts. The combination of these characteristics enables the triazole to evolve from being synthetic convenient to becoming an allosteric pharmacophore extension that enhances cell potency while maintaining molecular weight and lipophilicity levels.
Comparative Benefits
Where the design brief calls for a reversible solubility handle that is protonated within acidic vesicles and uncharged at blood pH, piperazine grants an off-the-shelf tunable pKa window by N-alkyl or N-acyl editing. Conversely, where the dominant shortfall is conformational entropy – that is, the ternary complex will not crystallize or the cellular DC50 plateaus – then the triazole's planar, rotation-locked scaffold bestows a zero-entropy hinge that frequently compresses the ligase–target interface independent of the warhead. Deciding between them is therefore less a matter of intrinsic potency than of which physicochemical liability most currently restricts the programme: piperazine favours solubility-driven pharmacokinetics, whereas triazole tips the balance towards entropic degradation efficiency. The following sections unravel these trade-offs in the form of recent literature vignettes that highlight when, and why, one heterocycle outperforms the other.
When to Choose Piperazine vs. Triazole?
Use of piperazine is rationalized once the first indications of precipitation of the lead PROTAC in FaSSIF media or irreversible adsorption to the syringe filter at sub-μM concentrations. Two nitrogen atoms in the ring may be differentially substituted: one side can be left basic, while the other is capped with a polar, even anionic, group, creating an internal "solubility cloak" without need for external excipients. Since basicity can be dialed down via distal carbonyls, the same core can be marched through a pKₐ range of roughly three logarithmic units without changing exit vectors, in a manner simply not possible with the electronically fixed triazole. On the other hand, triazole is favored when the crystallographic density maps indicate that the linker passes through a tight tunnel between E3 ligase and target protein: the heterocycle's planar geometry enforces a 120° angle that is close to that of a peptide bond, often using the same hydrogen-bond registry as a native amide, and therefore able to harvest enthalpic anchoring points that piperazine's flexible chair conformation cannot. Triazole is also favored when metabolic robustness is a priority: cytochrome P450 enzymes will oxidize tertiary amines to N-oxides or dealkylated fragments, while the triazole nucleus is essentially immune to phase-I transformations, which reduces risk of off-target metabolites with potential to mis-ubiquitinate innocent proteins. A pragmatic rule of thumb: call piperazine when the bottleneck is solubility or pH-triggered release; call triazole when the bottleneck is conformational heterogeneity or oxidative liability.
Case Studies in Literature
A recent study of BRD4 degraders showed the piperazine benefit in stark contrast. The first, based on a triazole-only linker, had low-nanomolar biochemical potency but was unmeasurably degrading in whole blood since the entire molecule precipitated out of solution at micromolar concentrations. Swapping the central triazole for a piperazine–PEG hybrid rescued solubility without increasing overall length, the optimized PROTAC displayed a magnitude better cellular DC50 and, critically, showed a linear expanse–response relationship in vivo across species, which is ascribed to the ring's protonation-dependent solubility in endosomes. The opposite lesson is recorded in an FKBP12 degrader campaign. In this instance, a flexible piperazine-rich linker endowed excellent solubility but gave shallow degradation kinetics; crystallographic data showed that the chair–chair interconversion of the piperazine enabled the warhead to sample a range of orientations, only one of which was favorable. Inserting a single 1,4-disubstituted triazole in the center as a rigid hinge pinned the dihedral angle, contracted the conformational ensemble, and sharpened the degradation curve without adding to the molecular weight. A third, more subtle example contrasts the two chemistries in the same molecule: a dual-degrader targeting BRD4 and FKBP12 used a piperazine on the solubilizing side and a triazole proximal to the E3 interface, thereby combining the pH-coupled solubility advantage of the former with the entropic concentration of the latter. The hybrid linker outperformed either homo-linker alone, emphasizing that the choice need not be mutually exclusive but can be made contextually and in sequence.
Buying Guide
At BOC Sciences, we specialize in supplying piperazine and triazole linkers-two of the most innovative classes driving the next generation of functional PROTAC chemistry. Our linkers are manufactured to the highest analytical standards, offering consistent performance and compatibility across a wide range of protein degradation platforms.
Pre-Validated Functional Linkers
All our piperazine and triazole linkers undergo rigorous validation to ensure excellent chemical integrity, purity, and reactivity. Each batch is delivered with a full Certificate of Analysis (COA) and analytical data package, including HPLC, LC-MS, and NMR profiles. We offer:
- Research-grade piperazine linkers optimized for solubility and conformational control.
- Click-ready triazole linkers synthesized via CuAAC chemistry for fast, modular PROTAC assembly.
- High purity and batch-to-batch consistency verified by in-house quality testing.
- Ready-to-ship inventory for rapid procurement and reduced lead times.
Our pre-validated linkers are trusted by medicinal chemistry teams worldwide to improve ternary complex formation, hydrogen bonding orientation, and bioavailability.
Custom Design Options
If your project requires a specialized functional linker, our custom synthesis service can design and produce linkers tailored to your exact molecular requirements. Customization capabilities include:
- Adjusting chain length, polarity, or rigidity through hybrid PEG-piperazine or triazole-aryl architectures.
- Incorporating specific end groups (amine, azide, alkyne, NHS, or carboxyl).
- Designing piperazine derivatives with tuned basicity or steric bulk for improved target-ligase compatibility.
- Isotopic or labeled variants for mechanistic and DMPK studies.
Our chemists collaborate directly with your R&D team to optimize both structure and synthesis feasibility. We provide rapid quoting, transparent timelines, and full analytical support with every delivery.
Partner with Us for Advanced Piperazine and Triazole Linkers
Piperazine and triazole linkers are redefining the design of functional PROTACs, offering structural precision, polarity control, and enhanced pharmacokinetic performance. As these linker types become the new standard in drug discovery, choosing a trusted supplier ensures consistent quality and faster project turnaround. At BOC Sciences, we combine cutting-edge synthesis, analytical rigor, and global logistics to deliver linkers you can rely on - whether you need off-the-shelf options or fully customized designs.
Contact our technical team today to request a quote, explore our functional linker catalog, or discuss a custom piperazine or triazole synthesis for your next PROTAC program. Advance your research with chemistry that's precise, innovative, and built for the future of targeted protein degradation.
FAQs
1. Why are piperazine and triazole linkers considered functional?
They provide polarity control, pKa tuning, and rigidity that enhance ternary complex formation.
2. Are triazole linkers compatible with click chemistry?
Yes, they are ideal for CuAAC reactions, ensuring efficient and modular conjugation.
3. Do you offer pre-validated linkers?
Yes, all functional linkers are analytically verified and supplied with COA and QC data.
4. Can I design a hybrid piperazine-PEG linker?
Absolutely—our custom synthesis team can tailor linkers for solubility, polarity, and orientation.
References
- Sun X, Gao H, Yang Y, et al. PROTACs: great opportunities for academia and industry[J]. Signal transduction and targeted therapy, 2019, 4(1): 64. https://doi.org/10.1038/s41392-019-0101-6.
- Zeng C, Avula S R, Meng J, et al. Synthesis and biological evaluation of piperazine hybridized coumarin indolylcyanoenones with antibacterial potential[J]. Molecules, 2023, 28(6): 2511. https://doi.org/10.3390/molecules28062511.
- Kubryń N, Fijałkowski Ł, Nowaczyk J, et al. PROTAC Technology as a New Tool for Modern Pharmacotherapy[J]. Molecules, 2025, 30(10): 2123. https://doi.org/10.3390/molecules30102123.
- Danishuddin, Jamal M S, Song K S, et al. Revolutionizing drug targeting strategies: integrating artificial intelligence and structure-based methods in PROTAC development[J]. Pharmaceuticals, 2023, 16(12): 1649. https://doi.org/10.3390/ph16121649.
- Distributed under Open Access license CC BY 4.0, without modification.

 Fig. 1 Mode of action of PROTACs.1,5
Fig. 1 Mode of action of PROTACs.1,5 Fig. 2 Design of piperazine hybridized coumarin indolylcyanoenones as new structural antibacterial agents.2,5
Fig. 2 Design of piperazine hybridized coumarin indolylcyanoenones as new structural antibacterial agents.2,5