PROTACs are widely used to degrade proteins of interest. In contrast to small molecule inhibitors, PROTAC can degrade any protein within the cell, including undruggable targets such as transcription factors and scaffold proteins. Although promising, conventional PROTACs generally have adverse pharmacokinetics and lack tumor specificity, which may lead to systemic toxicity due to their non-specific distribution in normal tissues. Therefore, achieving tumor-specific delivery and enhancing the anti-tumor efficacy of PROTAC remains a great challenge.
To achieve tumor-targeted delivery of PROTAC, several strategies, e.g. antibody-PROTAC, folate-caged PROTAC and aptamer-PROTAC conjugates, and poly-PROTAC nanoparticles, have been developed. These modified PROTACs showed better tumor accumulation and antitumor efficacy.
Building on the continued clinical and commercial success of ADCs with cytotoxic payloads, several studies have explored the coupling of PROTAC to monoclonal antibodies to improve their DMPK properties and enable higher delivery efficiency of PROTAC. The new molecule produced by antibody and PROTAC is called "DAC", which has several potential advantages over PROTAC molecules:
- It can deliver in vivo an inhibitor with poor physicochemical properties or DMPK properties;
- The avoidance of complex formulations that are usually necessary for PROTAC to become active in vivo exposure;
- Target PROTAC molecules of interest to specific tumors or tissues.
One of the earliest examples of DAC published in a peer-reviewed paper appeared in early 2020, describing a high-potency, VHL ligase-based degrader (GNE-987) for bromine domain protein 4 (BRD4) that binds to a targeting CLL1 antibody via a new cleavable linker containing disulfide bonds (Fig 1).
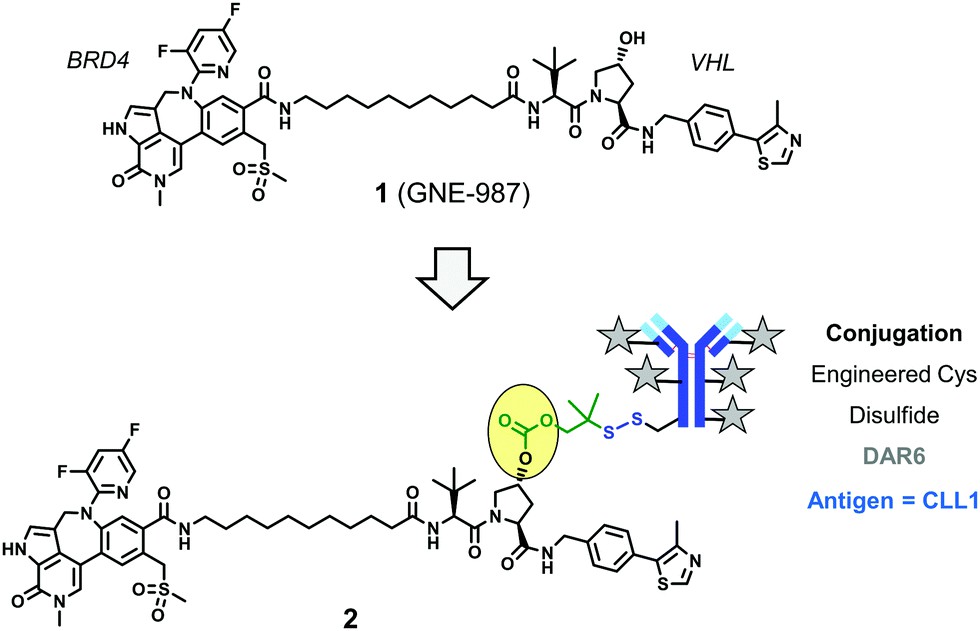 Fig 1. BRD4-targeting DAC, disulfide bond linker1
Fig 1. BRD4-targeting DAC, disulfide bond linker1
The DAC demonstrated a powerful, dose-dependent antitumor effect in vivo in a single intravenous administration of HL-60 and the EOL-1 acute myeloid leukemia (AML) xenograft model. In addition, controlled experiments showed that:
(1) CLL1 antibody without coupling with degrader had no in vivo activity;
(2) DAC carrying the GNE-987 hydroxyproline isomer payload was also ineffective;
(3) The corresponding DAC that recognized the HER2 antigen (not highly expressed on AML cells) had weak activity in vivo at the same dose;
(4) Uncoupled GNE-987 is inactive, and its poor PK characteristics may be the reason for this result.
Together, these results provide proof of concept that DAC can exert antitumor effects in vivo through ligase-dependent degradation mechanisms and in an antigen-dependent manner, and DAC can overcome the undesirable PK characteristics of PROTAC and achieve acceptable PROTAC lysosomal stability.
Folate receptor α (FOLR1) is the most well-defined target for drug delivery to cancer cells because FOLR1 is highly expressed in many cancer types, such as ovarian, lung, and breast cancer, and has very low or no expression in normal tissues or cells. In addition to FOLR1, other receptors, like FOLR2 and FOLR3, also deliver folate to cells but they have a lower affinity for folate than FOLR1. Therefore, the FOLR1 targeting strategy has been extensively studied and applied in tumor imaging and cancer-targeted drug delivery, and several FOLR1 targeting drugs are currently in phase II/III clinical trials.
These findings led researchers to develop the specific delivery of PROTACs to cancer cells using folate binding strategies to achieve controlled targeted degradation of the target protein, thereby eliminating the potentially harmful toxicity to normal tissue. To this end, researchers designed folate-caged PROTAC, which was preferentially transported to cancer cells with high FOLR1 expression. Upon entry into cancer cells, folate-caged PROTAC was catalyzed by intracellular hydrolase to release folate portions, and PROTAC recruited endogenous VHL E3 ubiquitin ligase to ubiquitinate POI for subsequent degradation by the 26S proteasome (Fig 2).
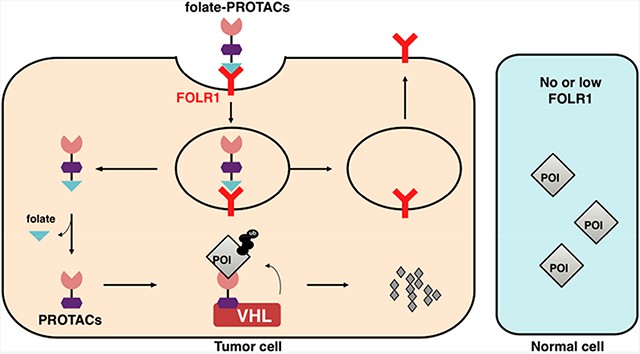 Fig 2. Folate-PROTAC mechanism2
Fig 2. Folate-PROTAC mechanism2
The study "Cancer Selective Target Degradation by Folate-Caged PROTACs" published in the journal JACS confirmed that folate-caged PROTAC, including BRD-PROTAC (folate-ARV-771), MEK-PROTAC (folate-MS432) and ALK-PROTAC (folate-MS99) can degrade BRD, MEK, and ALK, respectively, in a folate receptor-dependent manner in cancer cells. This design provided a scalable platform for PROTAC to achieve the selective degradation of POI in cancer cells.
The aptamer is a single-stranded nucleic acid with complex three-dimensional structures including stem-loop, hairpin, and G-quadruplex (G4) polymers. They bind to target proteins with high specificity and affinity through hydrogen bonding, van der Waals force, base stacking, and electrostatic interaction. Aptamer, also known as a chemical antibody, has significant advantages over other targeting vectors. For instance, the aptamer is suitable for large-scale preparation because it can be automatically synthesized through DNA solid phase. Besides, its structure is easy to modify to efficiently form several drug structures and improve water solubility. Furthermore, aptamer shows good tissue permeability and in vivo safety without significant immunogenicity.
Recent years witnessed the extensive applications of aptamer as a targeted therapy for human tumors. Aptamer AS1411 (AS) is rich in guanine bases and can specifically recognize and bind nucleolin, which is highly expressed on the tumor cell membrane and is widely used as a biomarker for tumor-targeted therapy. AS itself has good inhibitory activity against tumors with overexpression of nucleolin and is currently undergoing phase II clinical trials. In addition, AS is widely used as a transporter for the targeted delivery of small-molecule drugs to tumors.
Inspired by the promise of aptamer in targeted cancer therapy, experts developed a method to combine aptamer with conventional PROTAC to improve the tumor-targeting properties and antitumor efficacy of PROTAC. The study "Aptamer-PROTAC Conjugates (APCs) for Tumor-specific Targeting in Breast Cancer", described that the first aptamer-PROTAC conjugate (APC) was designed by modifying BET PROTAC with AS aptamer and a cleavable linker (Fig 3). The APC showed good specificity and effective BET degradation on MCF-7 breast cancer cells with high expression of nucleolin. Therefore, the APC strategy may provide a valuable tool to overcome the shortcomings of conventional PROTAC by improving its water solubility, tumor targeting ability, antitumor effectiveness, and reducing side effects in normal organs.
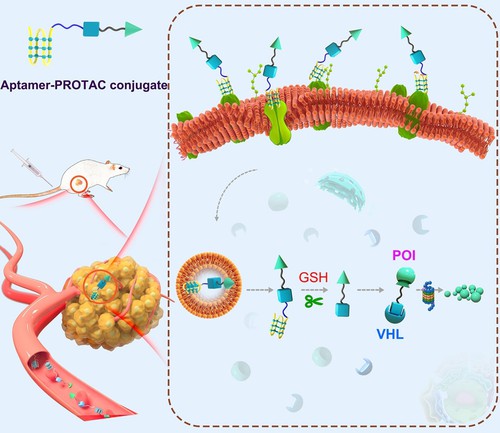 Fig 3. The design of AS-PROTAC conjugate3
Fig 3. The design of AS-PROTAC conjugate3
A research paper "Engineered bioorthogonal POLY-PROTAC nanoparticles for tumor-specific protein degradation and precise cancer therapy" was recently published in the journal Nature Communications. A poly-PROTAC nanoplatform activated by a tumor microenvironment was designed to achieve the tumor-specific antitumor properties of PROTAC.
For proof-of-concept purposes, poly-PROTAC nanoparticles were designed for targeting BRD4 (Fig 4). Researchers first synthesized four PROTAC small molecules based on VHL and then designed a series of reductively activated poly PROTAC and self-assembled them into micelle nanoparticles for PROTAC delivery. Subsequently, a pre-targeted nanoparticle loaded with dibenzocyclooctyne (DBCO) was designed to enhance the accumulation of azide-modified poly-PROTAC nanoparticles in the tumor by in situ click reaction. After internalization into tumor cells, poly-PROTAC nanoparticles released the PROTAC payload via glutathione (GSH) -mediated disulfide bond reduction. It was found that poly-PROTAC nanoparticles could synergistically induce tumor cell apoptosis when combined with photodynamic therapy (PDT) in a mouse model of MDAMB-231 breast cancer. This study thus provided a generic nanoplatform for tumor-specific PROTAC delivery and enhanced cancer efficacy.
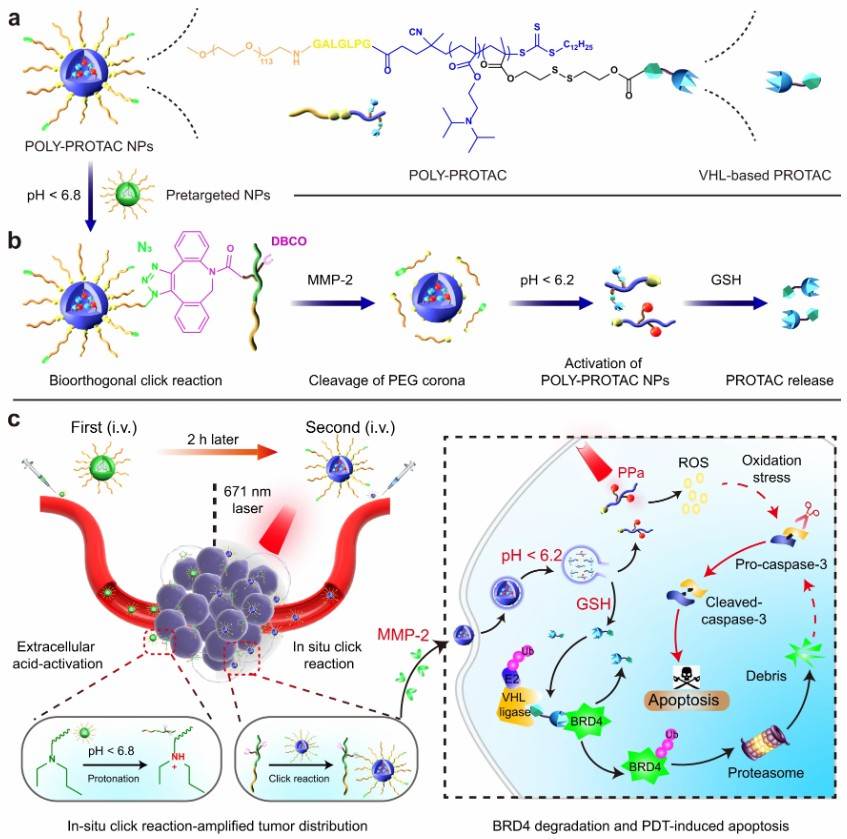 Fig 4. Poly-PROTAC nanoparticles4
Fig 4. Poly-PROTAC nanoparticles4
Conclusion
Accurate delivery of PROTAC to the tumor and effective degradation of POI within tumor cells remains a formidable challenge. Therefore, there is a great need to develop novel PROTACs for tumor-specific POI degradation while minimizing both on-target and off-tumor adverse reactions. Several studies have developed second-generation PROTAC to achieve more tumor accumulation, deeper tumor penetration, and stronger POI degradation performance. Although they are still in the preclinical stage, it is reasonable to expect that these strategies will soon translate into clinical benefits to better help patients.
References
- Peter S. Dragovich, Degrader-antibody conjugates, Chem. Soc. Rev., 2022,51, 3886-3897.
- Liu, J., et al. Cancer Selective Target Degradation by Folate-Caged PROTACs, J Am Chem Soc. 2021, 143(19): 7380–7387.
- He, S., et al. Aptamer-PROTAC Conjugates (APCs) for Tumor-Specific Targeting in Breast Cancer, Angew Chem Int Ed, 2021, 60(43):23299-23305.
- Gao, J., et al. Engineered bioorthogonal POLY-PROTAC nanoparticles for tumor-specific protein degradation and precise cancer therapy, Nature Communications, 2022, 13, 4318.

 Fig 1. BRD4-targeting DAC, disulfide bond linker1
Fig 1. BRD4-targeting DAC, disulfide bond linker1 Fig 2. Folate-PROTAC mechanism2
Fig 2. Folate-PROTAC mechanism2 Fig 3. The design of AS-PROTAC conjugate3
Fig 3. The design of AS-PROTAC conjugate3 Fig 4. Poly-PROTAC nanoparticles4
Fig 4. Poly-PROTAC nanoparticles4