Proteolysis targeting chimeras (PROTACs) is a rapidly developing field in drug discovery. PROTACs incorporate a ligand for the target protein, a linker, and an E3 ubiquitin ligase recruiting group, which makes PROTACs tend to have high molecular weight potentially. As a PROTAC-based technology that has emerged in recent years, in-cell click-formed proteolysis targeting chimeras (CLIPTACs) apply a new strategy to avoid the cell permeability limitation of pre-assembled PROTACs with high molecular weight.
As a leading provider of drug discovery and research services, BOC Sciences is committed to providing PROTAC development services. With the comprehensive platform and experienced expert team, we are capable of providing CLIPTACs design and optimization services to help our customers achieve their new drug discovery goals.
Introduction
PROTACs are bifunctional molecules that generally show poor bioavailability due to their relatively high molecular weight. Therefore, CLIPTACs have been developed to as an alternative method to treating cells with a high molecular weight PROTAC. CLIPTACs contain two smaller precursors that can be combined intracellularly by bioorthogonal reaction to achieve PROTACs in vivo synthesis. The promising bioorthogonal reactions involved in CLIPTACs include inverse electron demand Diels-Alder (IEDDA) cycloaddition, strain-promoted alkyne-azide cycloadditions (SPAAC) and Staudinger ligation, which are represented by the reaction of tetrazine-TCO, BCN-azide and arylphosphine-azide, respectively.
The ligands for E3 and target protein are generally small molecules that are likely to be much more bioavailable than PROTACs themselves. Therefore, the small molecule ligands have been designed to a pair of precursors capable of covalently binding in vivo, known as CLIPTACs. In this method, warheads of E3 and target protein are tagged by bioorthogonal reaction partners such as tetrazine- or trans-cyclooctene which are connected through IEDDA cycloaddition to synthesize bifunctional PROTAC molecules in the cell.
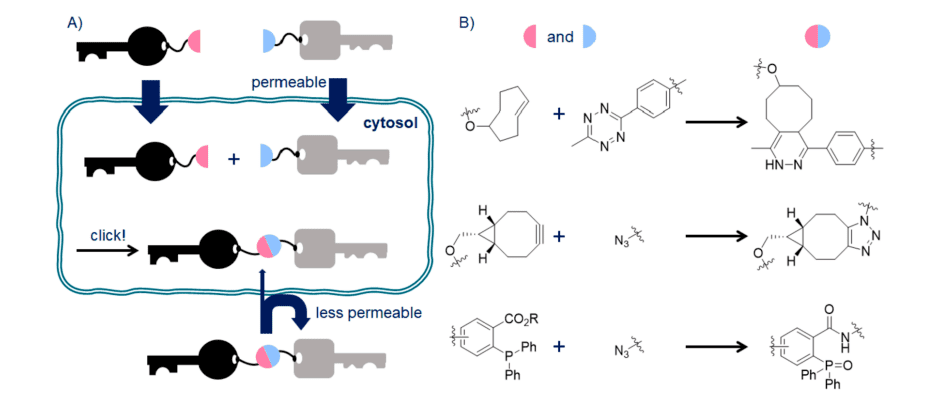 Fig 1. A) CLIPTAC. B) Bioorthogonal reaction partners in CLIPTAC.
Fig 1. A) CLIPTAC. B) Bioorthogonal reaction partners in CLIPTAC.
CLIPTACs Design
- Design of linking and tagging groups
The positions and lengths of the linking and click groups of two precursors need to be carefully designed. It has been shown that the length of the linker between the target protein ligand and the E3 ligase ligand plays a major role in determining the efficiency of protein degradation.
- Selection of bioorthogonal handles
Bioorthogonal reactions can take place in biological environments without affecting biomolecules or interfering with biochemical processes.
Characteristics of the bioorthogonal reaction:
- Biocompatible: The reaction occurs at the temperature and pH of the physiological environment.
- Selective: The reaction is not affected by the presence of endogenous nucleophiles and electrophiles in water or complex biological environments.
- Fast: The reaction occurs rapidly at low concentrations and forms stable reaction products.
The selection of suitable bioorthogonal handles is an important part of the design of CLIPTACs. For example, tetrazine-TCO cycloaddition that has been shown to be fast and high yielding without catalyst, has been successfully applied in the design of CLIPTACs showing activity in the target protein degradation.
Our Services
- Linker Design and Optimization
- CLIPTAC Precursors Synthesis
- CLIPTACs Evaluation
Our Advantages
- CLIPTACs design and optimization
- Well-established PROTAC platform
- Expertise and experienced scientific team
- Data analysis, detailed results reporting and discussion
- Highly reliable and reproducible results
Project Workflow
References
- Tomoshige, S., Ishikawa M., In vivo synthetic chemistry of proteolysis targeting chimeras (PROTACs), Bioorg. Med. Chem., 2021, 41, 116221.
- Lebraud, H., Wright, D. J., Johnson, C. N., and Heightman, T. D., Protein Degradation by In-Cell Self-Assembly of Proteolysis Targeting Chimeras, ACS Cent Sci., 2016, 2(12): 927–934.

 Fig 1. A) CLIPTAC. B) Bioorthogonal reaction partners in CLIPTAC.
Fig 1. A) CLIPTAC. B) Bioorthogonal reaction partners in CLIPTAC.
