In recent years, IAP-based PROTACs (SNIPERs) have evolved into a novel therapeutic approach for the treatment of many diseases. As a leading CRO, BOC Sciences is committed to building proteolysis targeting chimera platform. With our extensive expertise and integrated platform, we provide IAP-based PROTAC development services to help our clients in their drug discovery and development.
Introduction
IAPs are a class of anti-apoptosis proteins containing one or three baculovirus IAP repeat (BIR) structural domains, which regulate cysteinase and thus inhibit apoptosis. The IAP family consists of eight members, of which cIAP1 (cellular inhibitor of apoptosis protein 1) and XIAP (X-linked inhibitor of apoptosis protein) are the most widely studied. IAPs are overexpressed in a wide range of cancer cells and are strongly associated with tumor progression and poor prognosis, thus they have been validated as important targets for cancer therapy.
In addition, IAPs belong to the E3 ubiquitin ligase family. PROTACs based on the E3 ligase IAP are also known as specific non-genetic IAP-based protein erasers (SNIPERs), and IAP inhibitors and their derivatives are commonly used as E3 ligase ligands of SNIPERs. Currently, SNIPERs have been successfully used to degrade different target proteins associated with various diseases, including cancer, immune disorders and neurodegenerative diseases. More than 20 target proteins have been reported in research of IAP-based PROTACs, including AR, ER, BRD4, BCR-ABL, BTK, CDK, EGFR, JAK, etc. Therefore, SNIPERs technology is considered as a promising approach for disease treatment.
About IAP-based PROTACs
SNIPERs (IAP-based PROTACs) are designed to induce IAP-mediated ubiquitination and proteasomal degradation of target proteins. SNIPERs targeting different proteins (e.g., AR, ER, BRD4, BTK, CDK) usually have three basic components, ligands targeting pathogenic proteins and IAP inhibitors (E3 ligase ligands), and optimized linkers. Unlike classical PROTACs, IAP-based PROTACs have a dual function of degrading target proteins and IAPs, which facilitates the antitumor function, but it is also recommended that care should be taken in their design to avoid unintended side effects.
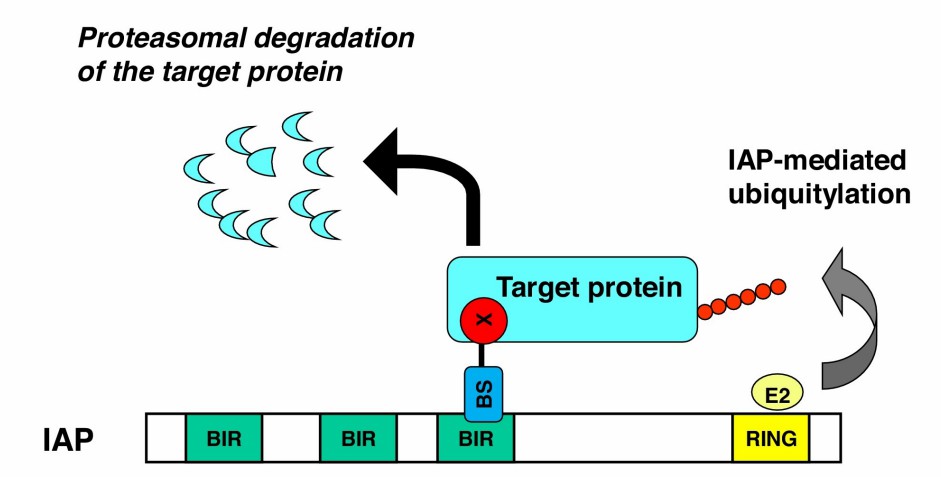 Fig. 1 IAP-mediated ubiquitylation and degradation of target proteins induced by SNIPERs. (Naito, 2019)
Fig. 1 IAP-mediated ubiquitylation and degradation of target proteins induced by SNIPERs. (Naito, 2019)
The SNIPERs use IAP inhibitors as targeting warheads. Since IAP mediates the development of multiple malignancies, IAP inhibitors are a highly promising therapeutic strategy for oncology. IAP inhibitors can act as E3 ligase ligands of SNIPERs, recruiting ubiquitin ligase cIAP1 or XIAP, which in turn leads to ubiquitination and proteasomal degradation of target proteins. IAP-binding ligands include bestatin, MV-1, LCL-161, A 410099, Cmpd37 derivatives, etc.
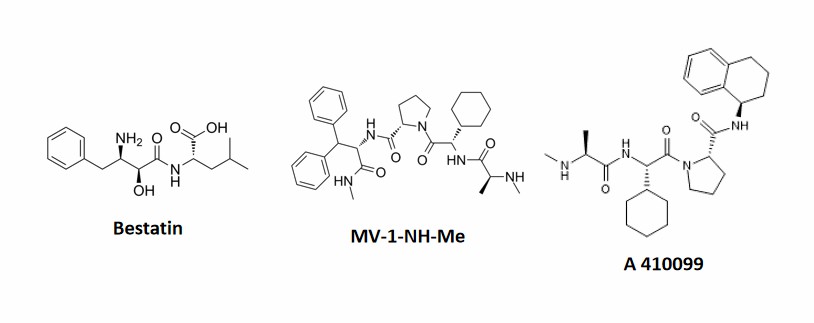
- The Target Proteins-binding ligands
Our Services
- Small molecular ligands design and screening
- IAP inhibitors synthesis
- IAP-based PROTACs design and synthesis
- Target-PROTAC-IAP ternary complex analysis
- IAP-based PROTACs activity assay
Advantages of IAP-based PROTAC
Compared to small molecule drugs, IAP-based PROTACs exhibit many advantages, including:
- Increased selectivity and specificity
- Overcoming drug resistance
- Degrading undruggable targets
Compared with PROTAC based on other E3 ligases (e.g. CRBN and VHL), IAP-based PROTACs can achieve dual-protein degradation of IAP and target proteins. Although degradation of the E3 ligase IAP itself may lead to inefficient degradation of the target protein, however, this is expected to counteract the adverse effects of IAP degradation due to the large amount of IAP continuously synthesized in the cell. Importantly, degradation of IAP can have a positive impact, as it has been reported that cancer cells typically overexpress IAP family proteins and that downregulation of IAP makes cancer cells sensitive to apoptosis.
Reference
- Naito, M., Ohoka, N. and Shibata N., SNIPERs—Hijacking IAP activity to induce protein degradation, Drug Discovery Today: Technologies, 2019, 31, 35-42.

 Fig. 1 IAP-mediated ubiquitylation and degradation of target proteins induced by SNIPERs. (Naito, 2019)
Fig. 1 IAP-mediated ubiquitylation and degradation of target proteins induced by SNIPERs. (Naito, 2019)
