PROTACs have demonstrated disease therapeutic potential by degrading various disease-related proteins. However, the off-target effect is one of the major limitations preventing the use of PROTACs in the clinic. Light-controllable PROTACs introduce a novel concept for persistent spatiotemporal control of induction of protein degradation, which may prevent off-tissue toxicity.
As a leading CRO, BOC Sciences is focused on the development of PROTAC technology. Based on our comprehensive platform and experienced expert team, we are committed to developing innovative alternative PROTACs that provide research tools and solutions for targeted protein degradation.
Introduction
The light-controllable PROTAC combines the concept of proteolysis targeting chimeric (PROTAC) and photochemistry that is called photopharmacology, which is an emerging field that uses light to precisely control drug activity in spatial and temporal resolution. PROTAC is a promising technology to target intracellular proteins including undruggable proteins targets for degradation. A PROTAC molecule is composed of three essential components as follows: a target protein binding ligand, a ligand of the E3 ligase, and a suitable linker that ensures the correct orientation of and the distance between the two ligands.
The first generation of PROTAC molecules were peptide-based, and their poor cell permeability and stability limited their use in medicine. With technological developments, second generation PROTAC molecules have been developed for the successful degradation of various proteins. In recent years, a third generation PROTAC with targeting delivery characteristic has been proposed. The light-controllable PROTAC is a one of the efficient strategies that can be activated by an extraneous cellular signaling (light) to achieve the spatiotemporal control of protein degradation, which should reduce toxicity, improve selectivity, and eliminate off-target effects.
Light-Controllable PROTACs Development
Recently, the development of light-controllable PROTACs has been receiving increasing interest. The photochromic and photocleavable functional groups are most extensively used in light-controllable PROTACs development.
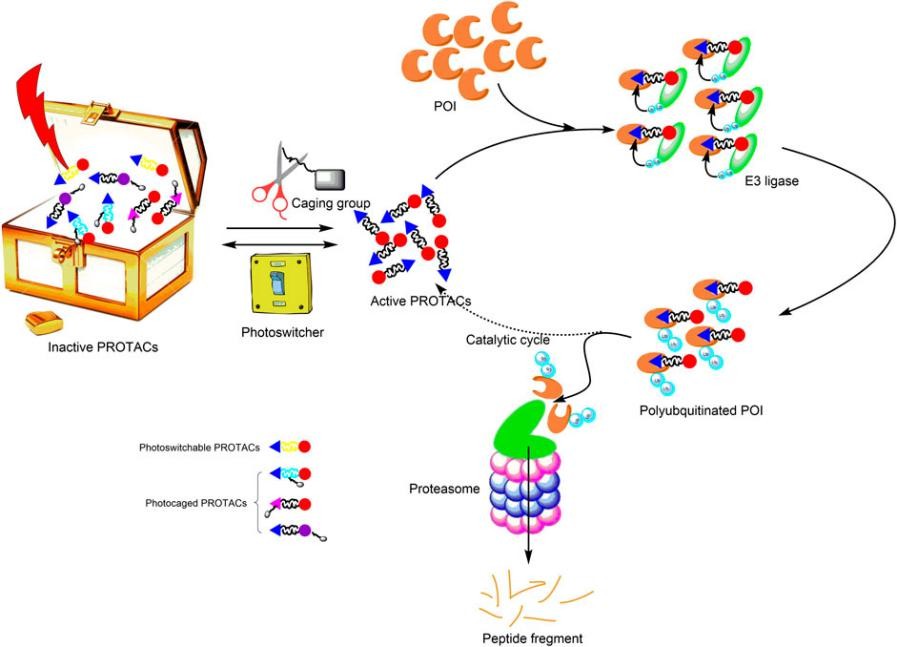 Fig. 1 Diagram of light-controllable PROTACs (Zeng, 2021)
Fig. 1 Diagram of light-controllable PROTACs (Zeng, 2021)
Building on the successful principle of bifunctional PROTAC, photoswitchable PROTACs (photoPROTACs) have been designed by introducing azobenzene linker between both warhead ligands. The trans-cis isomerization of azobenzene achieves reversible turning on and off of photoswitchable PROTAC molecular activity. The azo-trans-isomer is observed active because the proper distance defined by the azo-linker allows formation of the productive ternary complex formation. By contrast, the azo-cis-isomer is inactive because the linker is too short to engage both proteins in a ternary complex. A thermally stable photoPROTAC has been reported to generate the inactive cis-photoPROTAC under green light irradiation at 530 nm, and the active trans-photoPROTAC under blue light irradiation at 415 nm (Fig. 2). The bistable photoswitchable PROTACs offer a promising tool for novel therapeutics by protein degradation.
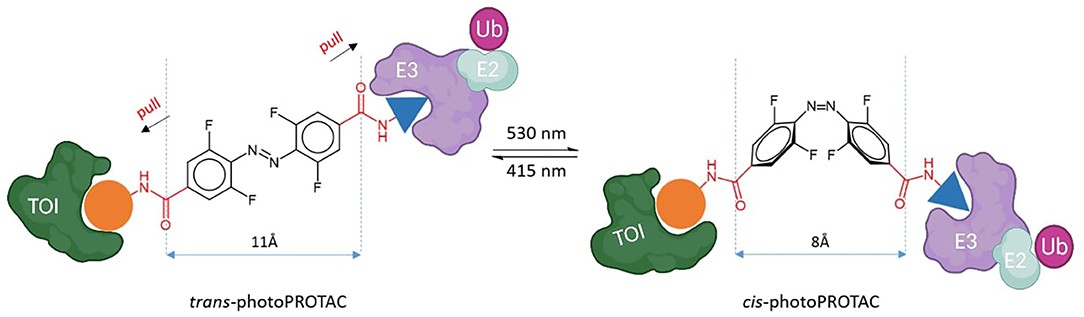 Fig. 2 trans-photoPROTAC and cis-photoPROTAC (Pfaff, 2019)
Fig. 2 trans-photoPROTAC and cis-photoPROTAC (Pfaff, 2019)
Photo-cage groups, also known as photoremovable protecting groups, provide the standard toolbox to spatially and temporally control the release of active molecules in vivo. Photo-cage PROTAC (pc-PROTAC) carries a photolabile protective group to prevent interaction with target protein or E3 ligases, which is irreversibly released upon light irradiation at certain wavelengths. With the development of biorthogonal chemistry, several photolabile groups for caging bioactive molecules have become a powerful tool for biological research. The photolabile groups enable the controllable activation of photo-cage PROTACs in target cells. The pc-PROTACs for inducing protein degradation with light expand the toolbox of disease-related protein target studies.
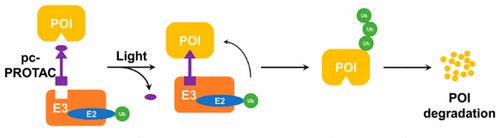 Fig. 3 photo-caged PROTACs (Xue, 2019)
Fig. 3 photo-caged PROTACs (Xue, 2019)
Our Services
- Targets ligands screening
- Light-controllable PROTAC molecules design
- Complex molecules synthesis
- Light-controllable PROTACs evaluation
Our Advantages
- Innovative PROTAC design
- Well-established PROTAC platform
- Expertise and experienced scientific team
- Data analysis, detailed results reporting and discussion
- Highly reliable and reproducible results
- Cost-effective and high-quality products
References
- Zeng, S., Zhang, H., Shen, Z., and Huang, W., Photopharmacology of Proteolysis-Targeting Chimeras: A New Frontier for Drug Discovery, Front. Chem., 2021, 10.
- Cecchini, C., Pannilunghi, S., Tardy, S., and Scapozza, L., From Conception to Development: Investigating PROTACs Features for Improved Cell Permeability and Successful Protein Degradation, Front. Chem., 2021.
- Xue, G., et al., Light-Induced Protein Degradation with Photocaged PROTACs, J. Am. Chem. Soc. 2019, 141, 46, 18370-18374.

 Fig. 1 Diagram of light-controllable PROTACs (Zeng, 2021)
Fig. 1 Diagram of light-controllable PROTACs (Zeng, 2021) Fig. 2 trans-photoPROTAC and cis-photoPROTAC (Pfaff, 2019)
Fig. 2 trans-photoPROTAC and cis-photoPROTAC (Pfaff, 2019) Fig. 3 photo-caged PROTACs (Xue, 2019)
Fig. 3 photo-caged PROTACs (Xue, 2019)