The choice of whether to base a PROTAC on cereblon (CRBN) or von Hippel–Lindau (VHL) is not merely a minor chemical nuance, but a critical design decision that can impact a PROTAC's pharmacodynamics, tolerability, and chemical space. CRBN ligands, such as pomalidomide, are a relatively small and orally available scaffold with a fast catalytic rate. However, they have an inherent off-target affinity for many zinc-finger transcription factors which may lead to additional immune-related effects. VHL ligands, which recognize a hydroxyproline pharmacophore, have a more buried binding pocket which leads to better selectivity for specific substrates, but at the cost of increased molecular weight and sometimes poorer cell permeability. The decision therefore depends on the particular design goals of the project, such as whether a high abundance of the ligase in tissues is most important, or whether avoiding off-target protein degradation in the hematopoietic system is a key priority, or whether there is a need for greater chemical space in the linker region for the PROTAC. Since neither ligase is clearly the best in all situations, the right answer is often dictated by the specific disease biology, target expression profile and risk tolerance of a project rather than by a strict preference for one scaffold or the other.
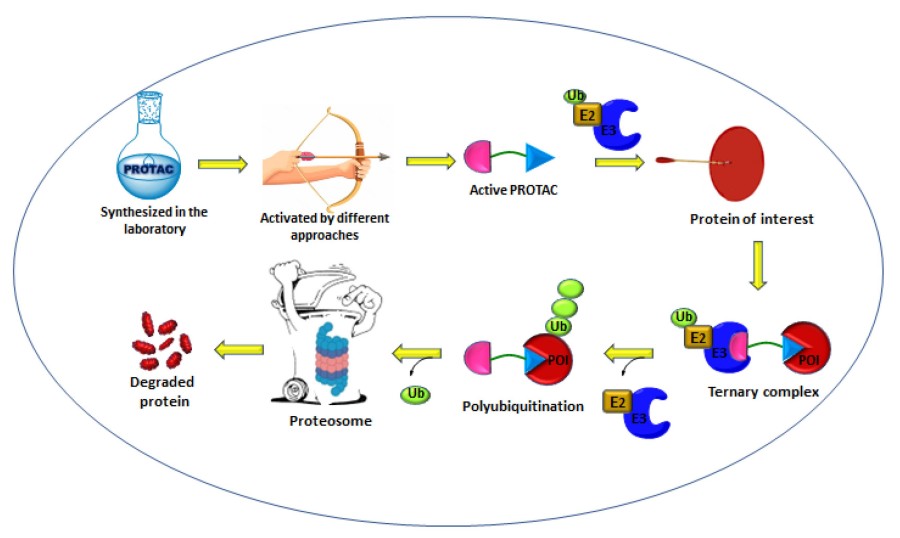 Fig. 1 Schematic of the mechanism of proteolysis-targeting chimeras (PROTACs).1,5
Fig. 1 Schematic of the mechanism of proteolysis-targeting chimeras (PROTACs).1,5
Why E3 Ligase Selection Is Critical?
E3 ligases represent a critical decision-making step in the ubiquitin–proteasome pathway, selecting which substrate proteins are to be ubiquitylated for degradation and which are to be spared. PROTACs hand over this critical decision-making process to a small molecule which must be a "fluent" in the language of a ligase and must also bind tightly to the protein-of-interest. A suboptimal pairing between a ligase and protein-of-interest will lead to plot impasse: insufficient expression of the ligase in the desired target tissue can lead to limited ternary complex formation and high enough binding affinity of a ligase to both protein-of-interest and E3 ligase can lead to excessive recruitment and off-target degradation. Such "toxic subplots" can often overshadow intended therapeutic effects. Moreover, the different subcellular localizations of E3 ligases (i.e. CRBN is primarily nuclear, whereas VHL is both cytoplasmic and nuclear) can also dictate which substrates are accessible for recruitment. Thus, ligase choice not only determines kinetic efficiency, but also determines the selectivity "lens" through which the degrader "manuscript" is "read" by the cell.
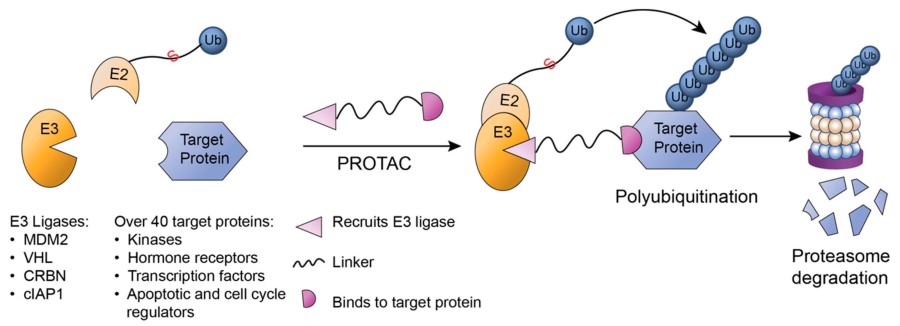 Fig. 2 Proteolysis targeting chimeras (PROTACs).2,5
Fig. 2 Proteolysis targeting chimeras (PROTACs).2,5
Overview of the Ubiquitin–Proteasome System
The ubiquitin–proteasome system (UPS) is thus like a library's archivist, reading every book looking for draft chapters and errata to shred. E1 enzymes first use ATP to adenylate ubiquitin, creating a high energy thioester bond that it transfers to an E2 conjugating enzyme. The E3 ligase here acts as the head-hunter, both recognizing the substrate to be destroyed and recruiting the ubiquitin-loaded E2~Ub E2 enzyme. By employing an induced-fit mechanism as well as proximity catalysis, the E3 enzyme helps the formation of an amide bond between the C-terminal glycine of ubiquitin and a lysine side chain on the substrate. Repeated cycles of this result in the formation of a K48-linked chain that will be recognized as a signal for degradation by the 26S proteasome. The proteasome is a barrel-shaped protease that unfolds and feeds the poly-ubiquitinated target protein into its catalytic core, simultaneously recycling the peptides and the ubiquitin itself. The hierarchy is important; E1s and E2s are fairly promiscuous, but the E3s give the pathway its specificity, providing an editorial voice. The human genome contains around 600 of these, each with its own set of recognition domains ranging from RING fingers to HECT and RBR catalytic cores. The relative numerical diversity gives the medicinal chemist a lot of handles to work with in the design of PROTACs, but the number of E3s with well-defined small molecule ligands is actually quite small, effectively shrinking the menu to CRBN, VHL, MDM2 and a few others. It is thus important to consider the kinetic phasing of a given ligase, how fast it loads ubiquitin, how long it sticks to substrate, how easy it is to recycle, in order to write the script for a degrader that can achieve pharmacology at low systemic exposure. The system has intrinsic homeostatic mechanisms as well; if the load of ubiquitination is too high, the ligase itself can be targeted for destruction and if proteasome is inhibited, there will be an up-regulation of E1 expression.
How Ligase Choice Affects Efficiency and Selectivity?
Ligase identity serves as an "on/off" switch to select the pages (proteome subsets) that are in focus. Due to its abundance in hematopoietic cells, CRBN-based PROTACs are particularly effective in achieving deep degradation of nuclear oncoproteins, but the broad substrate promiscuity of CRBN also "captures" zinc-finger transcription factors causing immunological side effects that can be dose-limiting. VHL-centred degraders, on the other hand, are enabled by a much smaller promiscuity window that gives high selectivity but at the cost of more limited tissue expression; VHL expression is relatively low in certain solid tumors and thus VHL-based PROTACs might need higher concentration to reach the threshold of ternary complex assembly. A related point is also that the kinetics of CRBN and VHL are different, and they may be more or less desirable depending on the cellular context and application. For instance, CRBN complexes have fast turn-over rates, which may be advantageous in situations where rapid protein degradation is needed (i.e., in rapidly dividing cells). In contrast, VHL forms relatively long-lived complexes that may be more suitable for targeting proteins that are more stable and need more persistent degradation signals. In addition, spatially localized ligases can only induce degradation of substrates at their locations; for instance, a nuclear ligase will not be able to induce degradation of cytosolic proteins (unless they possess nuclear localization signals). Finally, another important consideration, especially in regards to how quickly PROTACs make it to the clinic, is regulatory precedent. Regulatory agencies are likely to be more amenable to PROTACs based on ligases that have a long history of human safety data, such as CRBN, while PROTACs using ligases for which there are no approved IMiDs will require additional safety testing.
Structure and Function of CRBN and VHL
CRBN and VHL are two different design paradigms for ubiquitin enlistment. CRBN is a "pivoting door", a thalidomide receptor tethered to a Cullin-4 platform that closes upon ligand binding and "captures" transient neo-substrate interactions, materializing them into poly-ubiquitin chains. In contrast, VHL is a "pivoting clamp" in the elongin BC/Cul-2 complex, which recognizes hydroxylated proline motifs with high specificity. One platform selects for promiscuity, the other enforces a structural handshake. The folds of these E3 ligases, a β-sheet sandwich and an α-helix bundle respectively, reveal why the same PROTAC linker chemistry can drive opposite pharmacologies and why tissue microenvironments may prefer one ligase dialect over another.
Biological Pathways and Ligase Mechanisms
CRBN and VHL anchor pathways that intersect the domains of ion homeostasis and oxygen sensing, respectively. In the empty state, the CRBN box pores through cytosolic substrates, including endogenous ClC-2 channel regulator, to earmark them for degradation under altered membrane potentials. Occupancy of the small molecule binder induces an α-helical brace release, expands the entryway of the substrate portico, and misdirects neo-client substrates with β-hairpin degrons into the binding groove. Subsequent ubiquitination is mediated by the neddylation-regulated, Cullin-4 RING E3 module that is operatable in a transient manner to synchronize protein degradation to cellular metabolic states. VHL, in contrast, senses the oxygen status of the cell. In the presence of oxygen, factor hydroxylases hydroxylate a specific proline residue in hypoxia-inducible factors (HIFs); VHL binds to this hydroxy moiety to poly-ubiquitinate the transcription factor, repressing its hypoxic program. Oxygen deprivation arrests hydroxylation, VHL is released, and the program is transcribed. PROTAC designers capitalize on these natural designs: CRBN-based mechanisms are more likely to be tolerant of ligand-programmed redirects, while VHL-based designs are conditioned by the need for the target protein to display a hydroxy- or mimic- plug in an already select pocket. The two mechanisms further differ in their recycling kinetics: CRBN-based modules have a propensity for rapid turnover that is fit for resetting fast-paced signals, while VHL-based editing is of a slower tempo that correlates to its regulation by longer-lived stimuli. The understanding of these two stories guides PROTAC linker design and can help predict which cell state–proliferation, differentiation, stress–is likely to be most amenable to a given ligase.
Expression Profiles and Cellular Availability
CRBN mRNA is expressed ubiquitously in haematopoietic, neural and epithelial tissues. Expression is increased in activated lymphocytes and malignant plasma cells. PROTACs therefore have a large potential target window, but also are at risk of off-target degradation of zinc finger proteins. Phosphorylation near the C-terminus of CRBN has been shown to modulate the stability of the ligase, with higher levels being present during states of proteotoxic stress, and lower levels when cells are in a resting state. This should be considered when choosing a dosing schedule. VHL is expressed at the highest levels in the renal cortex, hepatocytes and vascular endothelium. Expression is moderate in solid tumors, and relatively low in peripheral blood immune cells. This could be leveraged to create PROTACs with tissue specific degradation, but would require higher doses if the target is present in tissues with low VHL expression. Trafficking is another consideration for availability, CRBN can shuttle between the nucleus and cytoplasm in response to ion changes, while VHL is predominantly cytosolic, bound to the elongin BC scaffold. This means that nuclear localization signals can be leveraged to direct CRBN-based PROTACs to nuclear targets. While VHL-based degraders may need cell permeable linkers to interact with cytoplasmic targets. VHL protein levels are also regulated by oxygen levels, with hypoxic tumors down-regulating the protein. This could impact degrader potency, and it may be necessary to use priming agents to up-regulate the ligase. These expression dynamics illustrate that the choice of ligase is not a static variable and should be chosen based on the target location, disease context and expected dosing regimen, in order to choose a ligase that has the right spatial and temporal expression pattern.
Chemical Characteristics and Binding Mechanisms
The CRBN and VHL anchors represent two orthogonal chemotypes that essentially speak different molecular languages. IMiDs are a planar glutarimide head that is H-bonded to a tri-tryptophan cage; the ligand is held by reversible π-stacking and electrostatic cuddling, and must induce a ligase conformational shift to bind. Hydroxyproline-based ligands, on the other hand, are wedged into a narrow VHL groove that enforces a defined hydroxyl stereochemistry that is similar to hypoxia-inducible factor, and are further stabilized by backbone amide complementarity and van-der-Waals bracing. The former is entropy-driven and kinetically labile, which is conducive to rapid catalytic turnover; the latter is enthalpically rich and more sustained, which enables exquisite selectivity but necessarily slower off-rates. By teasing apart the energetic grammars of these two anchors, we are better able to match the right anchoring language to the desired degradation story.
IMiD vs Hydroxyproline-Based Ligands
IMiD ligands are defined by a bipartite scaffold: a glutarimide ring that inserts into a hydrophobic pocket formed by β-sheets of CRBN and a phthalimide (or isoindolinone) appendage that remains solvent exposed and determines neo-substrate selectivity. Glutarimide carbonyls form hydrogen bonds with His380 and Trp382 and the aliphatic edge packs against a tryptophan triad, anchoring the IMiD in a rigid, planar fashion which can accommodate small electronic changes. Modifications to C4 on the phthalimide serve as an electronic handle: electron-donating amines, for example, can deepen π-stacking and expand the zinc-finger guest scope, whereas a carbonyl or nitrile introduces strain that closes the cavity and limits entry. The interaction is generally reversible, and residence time can be modulated with minor chemical changes rather than full scaffold redesign. As such, medicinal chemists can make use of an adaptable template for rapid PROTAC optimization. Hydroxyproline ligands are bound in a more defined lock-and-key fashion. The cis-4-hydroxyproline scaffold recapitulates a post-translational modification present on hypoxia-inducible factors and bidentately hydrogen bonds to Ser111 and His115 of VHL. This binding is decorated by a neighboring leucine or tert-leucine side chain which inserts into a hydrophobic groove, as well as an aromatic cap which engages Tyr98 in π-stacking. The resulting complex is enthalpically driven with slower off rates, which can lead to longer target residence at the cost of turnover. The hydroxyl stereochemistry is also more critical than in IMiDs, as epimerization to trans-hydroxyproline results in loss of affinity. Synthetic stereocontrol is therefore key in hydroxyproline synthesis. In terms of developability, IMiDs are often lower in molecular weight and have better oral bioavailability whereas hydroxyproline derivatives are more polar and higher in synthetic steps.
Ternary Complex Formation and Stability
The lifespan of a ternary complex is governed by both enthalpic contacts and entropic cost. IMiD-formed ternary complexes tend to be of low lifetime so that the ligase is free to recycle and enter another catalytic cycle after the ubiquitin transfer. The soft and compliant surface of CRBN is permissive of a diverse range of neo-substrate structures, however this may result in higher affinity to off-target partners. Therefore, linker length and exit vector must be engineered so that the target lysine is within the reach of the E2 active site without the ternary complex being too rigid. In contrast, hydroxyproline-VHL-formed ternary complexes tend to be more rigid in structure. The pre-organized hydroxyl-proline groove enforces a fixed angle between the ligase and target with less entropic penalty, but epitope matching is stricter. The cooperativity, or the increase in apparent affinity when all three complex partners are present, is expected to be greater for VHL-based complexes, as the enthalpic contribution of the hydroxyproline lock is higher. On the other hand, too much rigidity in the ternary complex may compromise the ubiquitin processivity if the target lysine is trapped at a distance outside of the catalytic radius. To address this, linker or kinked glycol segments are introduced into the structure to regain some conformational breathing. In general, the thermodynamic signature of the ternary complex must be matched to the biological half-life of the target protein to design an efficient and selective degradation pathway.
Advantages and Limitations of Each Ligase
CRBN and VHL are better thought of as "complementary but different tools" than as "interchangeable wrenches." CRBN provides a small, orally available glutarimide key that binds fast to the Cullin-4 lock, allowing for quick catalytic turnover and widespread tissue distribution; but that same keyhole is also open to zinc-finger "skeleton keys" that could trigger immunological off-target effects. VHL, by contrast, sports a stereochemically more picky hydroxyprolane ward which only opens to clients carrying the appropriate hydroxyl-badge, offering more fine-tuned selectivity at the price of larger molecular weight and sometimes slow cellular import. Neither ligase is better for all indications—they are only better if the disease context, target distribution and risk appetite of the regulatory environment match their underlying biophysical properties.
When to Choose CRBN-Based Systems?
CRBN should be considered as first-in for the programme where speed of degradation and rapid scalability across tissue compartments is required. Ubiquitously expressed in hematopoietic and epithelial tissues, CRBN supplies a ready pool of active surfaces without the need for target engagement-dependent transcriptional upregulation. The highly promiscuous ligand-binding groove accommodates a broad spectrum of neo-substrate topologies, from β-hairpins and unstructured loops to more rigid zinc-finger clusters. This versatility bodes well for onco-targets where no discrete hydrophobic binding pocket exists. The glutarimide pharmacophore is synthetically minimal, and C4/5 modification by amide/carbamate/urea exit vectors can be achieved under mild conditions, allowing for rapid linker scanning while ligase affinity remains unchanged. The short dwell times of CRBN also allow the same PROTAC molecule to be recycled for multiple rounds of catalysis, enabling lower doses and decreased off-state liabilities (e.g. ligand sequestration). Another reason CRBN can often serve as the starting point for new programmes is due to its favorable regulatory history: with decades of IMID clinical safety data, there is a ready-made toxicological dictionary with which regulators are comfortable and require limited new supporting sections. Finally, if the target of interest is nuclear and transcriptionally amplified, and is expressed in a tissue where CRBN density is high (e.g. lymphoid tissue, bone marrow, some solid tumors), this also bodes well for efficient ternary complex formation without the need for specialized delivery systems. In summary, CRBN is a good place to start when speed of action, ease of manufacture, and wide tissue penetration are at a premium compared to potential off-target effects on zinc fingers, and when moderate selectivity is acceptable in exchange for an accelerated translational programme.
When VHL Outperforms in Cellular Contexts?
VHL-based degraders are worth considering if your therapeutic narrative requires high selectivity and your target only exists in VHL-rich environments (renal cortex, liver, or vascularised tumour cores where VHL is endogenously expressed at high levels). The hydroxyproline code is quite rigid: the substrate must have a cis-4-hydroxyproline mimic or a stereochemically analogous mimic in order to fill the ternary complex. Thus, non-target binding zinc-finger proteins will be largely excluded. Such selectivity is useful for proof of concept applications where off-target degradation may obfuscate the desired mechanism. They also tend to have longer residence times than CRBN ligands, which is useful when the target is unusually stable or buried in protein complexes that require more time for ubiquitylation in order to be targeted for proteasomal destruction. Stereochemical control also provides a convenient control for medicinal chemists: the opposite epimer of the hydroxyproline will bind but will not trigger degradation. Such matched-pair comparisons are useful during mechanism of action reviews, for which regulatory agencies are fond. In addition, VHL ligands are typically less aggregation-prone despite the larger molecular weight of the linker, since these ligands generally have intramolecular hydrogen bonding interactions. In some cases, this can help with solubility issues experienced by CRBN ligands. The fact that VHL is a tumour suppressor also reduces the risk of resistance by loss of the ligase, which is nice for the overall story. Consider VHL if the target is cytoplasmic or membrane proximal, if neo-substrate presentation can satisfy the more rigid hydroxyproline requirements, and if the increase in selectivity outweighs the synthetic cost and potential loss of cellular uptake.
Practical Selection Guidelines
In many ways, selecting between CRBN and VHL anchors isn't so much a question of belief as it is an optimization question where the ‘correct' answer depends on the target, the cell cycle phase, the desired kinetics etc. Rather than postulate that one anchor is superior, an effective approach may be to set up a weighted-score matrix that evaluates not only ligase availability/tissue expression maps, neo-substrate size/drugability, known successes/efficacy or any other attributes that might be important, then to analyze specific cases. We find that many of these properties that may seem to be at odds with one another—kinetics, potency, on-target vs. off-target activity, oral availability vs. molecular weight—can be traded one against another to a certain extent by e.g. editing the linker or fine-tuning the dosing, once the decision-making process is clarified. Here are two more lenses to highlight a few of the most common scenarios.
Factors to Consider: Target Type, Cell Line, and Drug-Like Properties
The first decision in the triage is based on the intended target topology. Transcription factors with solvent-accessible β-hairpins or zinc-finger loops are obvious substrates for CRBN, while scaffolds with cytoplasmic localization and hydrophobic pockets suitable for hydroxyproline mimicry are amenable to VHL. For proteins that shuttle between compartments, one should ask where the pathology resides; a strong nuclear localization bias swings the decision towards CRBN, as does an active site buried deep from the membrane. The second vertex is cellular context: it is prudent to interrogate public expression databases for the ratio between target and E3 ligase abundance to avoid the surprise of the target being highly expressed while the ligase of choice is rare. E3 ligase expression in the functional assay should take this into account, with proof-of-concept experiments using a ligase-overexpressing system in a low-background host but confirmation in patient-derived cells or tissues containing the endogenous levels of the E3 to avoid false positives due to artifically-inflated potency. The last factor is chemical space. CRBN ligands have a smaller inherent contribution to molecular weight and lipophilicity, affording more space for linkers or large target mimics while maintaining a drug-like profile, while VHL ligands come with a larger mass and polar surface area, albeit balanced in part by the hydroxyproline hydroxyl as a handle for aqueous solubility and lower membrane affinity.
Hybrid and Dual-Ligase PROTAC Designs
In addition to targeting proteins for degradation in heterogeneous tissues, or in order to overcome potential resistance to ligase-loss, hybrid designs have also emerged in which both CRBN and VHL ligands are integrated in one degrader molecule. The idea behind such molecules is to use a branched or photoswitchable linker to occupy one ligase at a time, effectively using the degrader as a "switch" between the two ligases. This is immunized against variability in the expression of one of the two ligases (e.g. if CRBN becomes less expressed in the hypoxic center of a tumor, the VHL-tag can still recruit an effective ternary complex). Usually, these are synthesized by a late-stage orthogonal conjugation approach, in which a rigid aryl core is equipped with orthogonal protecting groups that allows the stepwise attachment of the two ligands, which is performed in a way that ensures that the stereochemistry of the hydroxyproline remains untouched (i.e. does not racemize) and palladium-free. Moreover, the positioning is often designed in such a way that only one of the ligases can be productively bound at a time. This is to avoid the formation of non-functional "dead-end" complexes that would sequester both pools of adaptor. In addition to such true hybrids, the term "dual-ligase" is also used for other strategies that include both VHL and CRBN ligands, such as dosing CRBN-based degraders on one day and VHL-based degraders on the next, which can be used to reduce on-target off-tumor toxicities while maintaining effective target inhibition. Such approaches are interpreted as "combination therapy in one pill" by regulators, requiring orthogonal safety assessment for both ligase arms; however, the advantage of being able to use lower overall doses to achieve the same pharmacology can outweigh the additional toxicology burden. Switching between one ligand and another can also be achieved by photocaged branches, where ultraviolet light selectively cleaves one of the two ligands to commit the degrader to a monovalent life only within the illuminated tissue.
Our Ligand Portfolio and Technical Support
Available CRBN and VHL Ligands in Analytical and Bulk Grades
We provide a comprehensive portfolio of CRBN- and VHL-based ligands in both analytical-grade and bulk-scale quantities, suitable for everything from early-stage PROTAC design to preclinical manufacturing. All ligands are synthesized under strict quality control with verified purity ensuring consistent biological performance and reproducibility in degradation assays. Each batch is supported by complete analytical documentation-including HPLC, LC-MS, and NMR data-allowing seamless integration into regulatory and internal QC pipelines. Whether you are conducting mechanistic studies, SAR evaluation, or large-scale screening, our ligands deliver dependable results backed by scientific precision. From standard pomalidomide-based CRBN ligands to hydroxyproline-derived VHL ligands, we supply the building blocks you need to accelerate PROTAC innovation.
Custom Synthesis for Novel Ligase Ligands
For teams developing next-generation E3 recruiters or exploring new ligase targets, we offer fully customized synthesis services. Our experienced chemists can:
- Modify existing CRBN or VHL scaffolds to improve binding affinity or solubility.
- Design and produce novel E3 ligase ligands based on emerging literature or proprietary targets.
- Introduce tailored linker handles at precise chemical positions to maintain ternary-complex stability.
We support structure-activity-guided optimization, helping you balance reactivity, selectivity, and metabolic stability for your chosen PROTAC system. With in-house synthesis, analytical validation, and scale-up capability, we turn your ligand concept into a research-ready material quickly and reliably.
Technical Assistance: Screening, Optimization, and Scale-Up
Our scientific team provides end-to-end technical support across the entire PROTAC development cycle. From ligand selection and in silico modeling to experimental screening and process optimization, we help researchers achieve optimal degradation profiles efficiently. We also assist with scale-up feasibility, purification strategy design, and stability testing, ensuring your ligase ligands transition smoothly from bench to pilot production. Clients benefit from direct access to senior chemists and a collaborative R&D workflow tailored to project needs. Whether you need data-driven screening guidance or commercial-scale preparation, our support ensures reliability and reproducibility at every stage.
Partner with a Trusted Supplier for E3 Ligase Ligands and PROTAC Support
Explore our curated catalog of high-purity CRBN and VHL ligands, linkers, and PROTAC building blocks. Each entry includes technical datasheets, structural details, and analytical specifications to simplify your compound selection process. Browse now to identify the right E3 ligase ligand for your PROTAC research or development pipeline.
Ready to move beyond off-the-shelf solutions? Collaborate with our R&D chemistry team to co-design, synthesize, and validate custom PROTAC reagents tailored to your target protein or E3 strategy. We offer rapid response times, clear project timelines, and full technical transparency. Get in touch today to discuss your PROTAC project requirements and leverage our expertise in E3 ligase chemistry and custom ligand development.
FAQs
1. What is the main difference between CRBN and VHL in PROTACs?
CRBN-based systems rely on IMiD ligands, while VHL uses hydroxyproline scaffolds. CRBN provides versatility; VHL offers superior selectivity and stability.
2. How do I choose between CRBN and VHL ligases?
Selection depends on your target protein, expression profile, and cell type-CRBN is broadly expressed, whereas VHL is ideal for tissues with low CRBN activity.
3. Can both ligases be used in hybrid PROTAC designs?
Yes, dual-ligase PROTACs are being developed to improve degradation efficiency and reduce resistance.
References
- Tamatam R, Shin D. Emerging strategies in proteolysis-targeting chimeras (PROTACs): highlights from 2022[J]. International Journal of Molecular Sciences, 2023, 24(6): 5190. https://doi.org/10.3390/ijms24065190.
- Liu Q, Aminu B, Roscow O, et al. Targeting the ubiquitin signaling cascade in tumor microenvironment for cancer therapy[J]. International journal of molecular sciences, 2021, 22(2): 791. https://doi.org/10.3390/ijms22020791.
- Cieślak M, Słowianek M. Cereblon-recruiting PROTACs: Will new drugs have to face old challenges?[J]. Pharmaceutics, 2023, 15(3): 812. https://doi.org/10.3390/pharmaceutics15030812.
- Liu J, Peng Y, Wei W. Light-controllable PROTACs for temporospatial control of protein degradation[J]. Frontiers in Cell and Developmental Biology, 2021, 9: 678077. https://doi.org/10.3389/fcell.2021.678077.
- Distributed under Open Access license CC BY 4.0, without modification.

 Fig. 1 Schematic of the mechanism of proteolysis-targeting chimeras (PROTACs).1,5
Fig. 1 Schematic of the mechanism of proteolysis-targeting chimeras (PROTACs).1,5 Fig. 2 Proteolysis targeting chimeras (PROTACs).2,5
Fig. 2 Proteolysis targeting chimeras (PROTACs).2,5