There are many differences between different drug delivery systems in the loading mode, release behavior, and delivery route of PROTAC. According to the assembly methods between PROTAC and drug delivery vector, PROTAC preparations can be classified into two different types, including PROTAC-oriented delivery system and PROTAC concept-inspired delivery system.
1 PROTAC-oriented delivery systems
For the delivery systems with PROTAC as the core, the goals are to increase solubility, enhance cell internalization, promote focal site accumulation, and reduce side effects. Based on the interaction between PROTAC and the delivery system, the preparations can be further classified into two categories: the PROTAC physically encapsulation delivery system and the chemical coupling PROTAC delivery system.
1.1 PROTAC physical encapsulation delivery systems
Several delivery systems physically encapsulate PROTAC with covalent or non-covalent ligands, alkyl, or PEG linkers. These delivery systems are capable of efficiently loading small hydrophobic compounds and delivering them to target locations. In addition to lipid nanoparticles, polymer nanoparticles, and protein-based delivery systems, mesoporous silica, metal-organic frameworks, and hydrogels also have the potential to deliver PROTAC. These vector materials can be used as therapeutic agents or excipients, and their safety and efficacy have been demonstrated through clinical trials.
In different delivery systems, PROTACs are primarily encased in lipophilic substrates, compartments, or cavities. They are also formed by monomers or aggregates of the carrier material through hydrophobic, electrostatic, hydrogen bond interactions, van der Waals forces, and π-π stacking forces. Once the delivery process is complete, the degrader dissolves, diffuses, or releases with the dissociation of the carrier material. Given the relative ease of adjustment of these advanced dosage forms, the addition of specific sensitive groups to the carrier may result in a responsive release of PROTAC. Overall, these PROTAC delivery systems are one direction that can help protein degraders achieve clinical transformation, given their low toxicity, biodegradability, and high delivery efficiency.
1.2 Chemical coupling PROTAC delivery systems
Unlike physical packaging, the chemical coupling is only suitable for PROTACs with modifiable groups, such as amines, carboxyl groups, and hydroxyl groups. Theoretically, these PROTACs can be bound to a variety of carriers or biomaterials, including small molecules, antibodies, peptides, aptamers, polymers, and nanoparticles. In addition, an important component of the chemically constructed PROTAC delivery system is the linker that connects the PROTAC to the carrier molecule. The linker must remain relatively stable during transport, at the same be cleavable to allow the effective release of PROTAC when internalized into subcellular organelles.
Therefore, many vector linkers are designed to respond to specific enzyme or environmental changes and thus trigger hydrolysis reactions. Some are also designed to be sensitive to exogenous signals such as ultrasound, magnetism, and radiation, or endogenous stimuli including acidic environments, REDOX stress, and hypoxic conditions. Once PROTAC is bound to a vector or group, their physicochemical properties including activity usually change, which allows PROTAC to overcome defects such as low cell permeability, poor pharmacokinetic characteristics, poor biological distribution, and side effects. However, the chemical coupling PROTAC delivery strategy has some limitations.
By utilizing the interaction between electrophiles and nucleophiles, PROTAC with reversible covalent bonds can also be connected to the delivery system without a linker. For example, many biological proteins and synthetic biological materials used as carriers have free sulfhydrylthat can be used as soft nucleophilic reagents to react with cyano-acrylamide and other soft electrophiles. Therefore, PROTACs with a reversible covalent motif can form covalent bonds with vector molecules to transport and release PROTAC in acidic microenvironments. In general, for PROTACs containing reversible covalent ligands of E3 ligases or target proteins, researchers may be able to utilize biomaterials containing nucleophiles as delivery vehicles.
2 PROTAC concept-inspired delivery system
The basic mechanism of PROTACs is the bifunctional chimera-mediated interaction between E3 ligase and POI, which shortens the spatial distance between an E3 ligase and the target protein and causes the proteasome system to degrade the target protein. In fact, in addition to small molecules, many delivery system components, such as peptides, nucleotides, protein fragments, polymers, and even nanoparticles, can also capture or bind certain proteins and E3 ligases. Furthermore, the spatial structure of the delivery system provides a platform for the proximity of E3 ligase and POI.
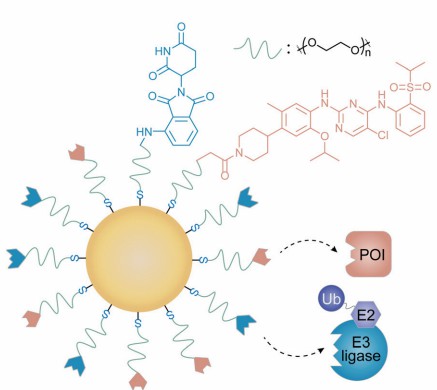 Fig 1. The depressant nanoparticles Cer/Pom-PEG@GNP1
Fig 1. The depressant nanoparticles Cer/Pom-PEG@GNP1
By combining ligands of CRBN and anaplastic lymphoma kinase (ALK) into gold nanoparticles, Wang's group obtained a PROTAC-inspired nanoparticle to overcome the potential drug resistance and off-target effects of small molecule inhibitors. Using the interaction between sulfur and gold, they used thiol-modified flexible PEG as a filler to attach the adhesive to the gold nanoparticles. The resulting gold nanoparticle-based PROTAC, called Cer/Pom-PEG@GNP, contains the ALK-bing ceritinib and the E3 ligand pomalidomide. Because Cer/Pom-PEG@GNPs have the ideal size and positive surface potential, they can be effectively internalized by NCI-H2228 cells.
In addition, the couplings on the surface of gold nanoparticles revealed good stability under different physiological simulation conditions. In co-incubation with NCI-H2228 cells, Cer/Pom-PEG@GNP showed effective ALK degradation and produced specific cytotoxicity. Compared with pure chemical PROTAC, this gold nanoparticle target protein depressant exhibited ideal solubility, stability, and half-life. In conclusion, the combination of PROTACs and drug delivery systems makes the two complementary advantages, and the design of new drug delivery systems inspired by the PROTAC mechanism also expands the research frontier of delivery systems.
3 Delivery systems expand applications of PROTACs
In the case of solid tumors, these diseases often present a variety of challenges for PROTAC-based therapies due to their complex tumor microenvironment, resistance mechanisms, and heterogeneity. The combination of PROTACs and other conventional therapies with the advantages of synchronous delivery, synergistic therapy, and controlled release will also be an emerging trend in the treatment of these complex diseases. Zhang's research group polymerized PROTAC fragments and synthesized semiconductor polymer nanoparticles with the help of cathepsin B cleavable peptide. The nano-PROTAC, named SPN pro, combined phototherapy with controlled protein degradation for photoimmunometabolic cancer therapy. The IDO-targeted PROTAC peptide contained the widely used IDO inhibitor NLG919, linker succinic acid, and VHL-binding peptides designed to re-engineer the immunosuppressive microenvironment. Polyethylene glycol semiconductor polymers with good biocompatibility and adjustable optical properties were used as phototherapy components. Due to the smaller PEG fragment size and stronger hydrophilicity, the circulation time of SPN pro in vivo was prolonged and can accumulate gradually in the primary tumor tissue.
After being internalized by cancer cells, SPNpro was activated by cathepsin B and rapidly released PROTAC, which effectively reduced the expression level of IDO in cells through the proteasome pathway involving VHL. Twenty-four hours after the administration of nanoparticles in 4T1 tumor-bearing mice, the immunogenic phototherapy was triggered by near-infrared light (NIR) irradiation at the primary tumor lesion site, which acted synergically with IDO-targeted PROTAC to enhance T cell antitumor immunity.
In addition, Zhang's team used similar polymer nanoparticles to deliver PROTAC targeting cyclooxygenase 1/2 (COX1/2) to regulate the immunosuppressive tumor microenvironment and promote tumor immunotherapy. These studies highlight the unique advantages of nanoparticle-mediated phototherapy, immunotherapy, and PROTAC integration.
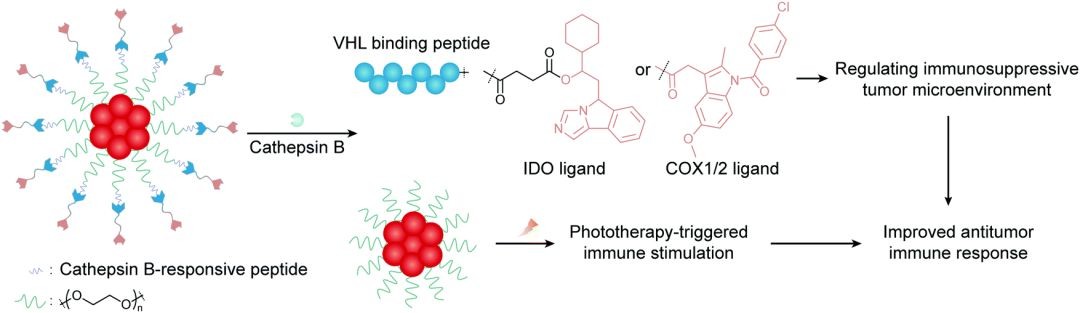 Fig 2. The combination therapy of PROTACs, phototherapy, and immunotherapy mediated by polymerized nanoparticles2
Fig 2. The combination therapy of PROTACs, phototherapy, and immunotherapy mediated by polymerized nanoparticles2
The antibody-PROTAC polymer enables simultaneous POI degradation and antibody-mediated tumor-specific membrane protein inhibition in combination therapy. For example, BRD4-targeting PROTAC combined with trastuzumab can simultaneously block HER2 and degrade BRD4. In addition, a PROTAC-loaded hydrogel delivery system, such as GT20029 formulated as a gel, enables a dual therapy of local administration combined with surgery. PROTACs are generally considered very promising in the treatment of neurodegenerative diseases, cardiovascular diseases, autoimmune diseases, and malignant tumors. However, given its rapid blood clearance in vivo and other adverse pharmacokinetic parameters, PROTAC is difficult to achieve adequate concentrations at the disease site during actual administration, which can lead to increased frequency of administration and adverse reactions. Targeted drug delivery systems with extended cycle time or controlled release mechanisms can reduce the required PROTAC dose and improve compliance in patients requiring long-term medication.
For example, Luo's group recently designed an HMGCR-targeted PROTAC for the treatment of hypercholesterolemia. The bioavailability of PROTAC was improved by designing PROTAC into a lactone prodrug. Oral administration of the modified PROTAC significantly improved plasma exposure to PROTAC and reduced cholesterol content in mice with atherosclerosis. This finding suggested that a suitable delivery system or formulation could allow PROTACs to maintain high bioavailability even after being taken orally. In addition to improving its adverse pharmacokinetic properties, the delivery system can also reduce the frequency of administration by extending PROTAC activity time after the introduction of a responsive release mechanism.
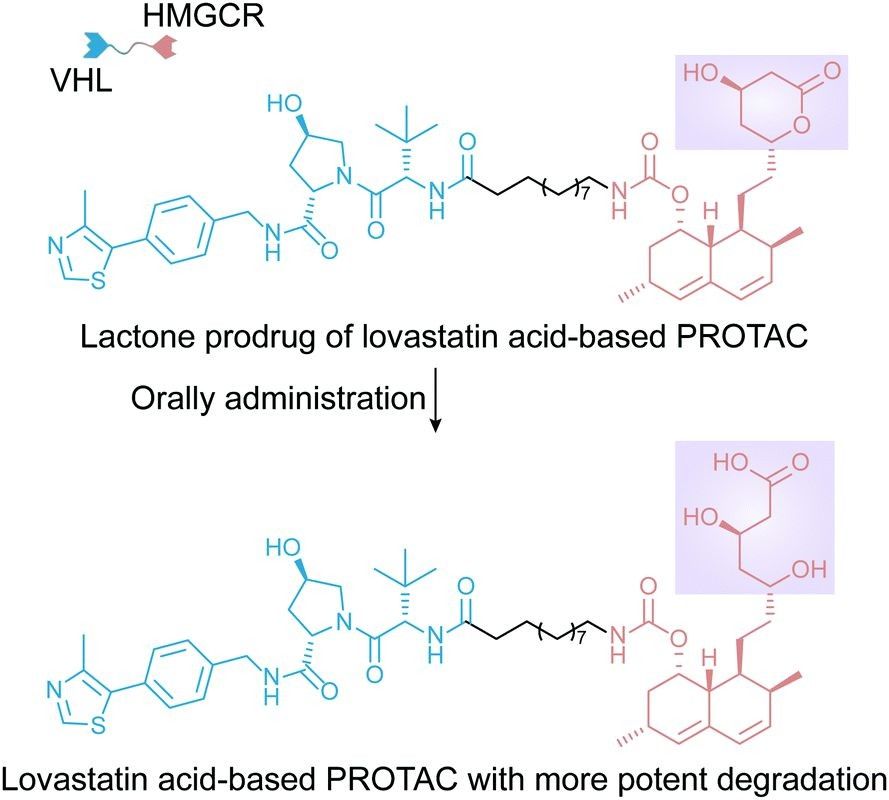 Fig 3. The structure of HMGCR-targeting PROTAC3
Fig 3. The structure of HMGCR-targeting PROTAC3
4 Conclusion
Although great progress has been made in PROTAC delivery, some challenges remain. First, additional vector materials and medicinal excipients may increase manufacturing costs associated with PROTAC. Secondly, the safety of PROTAC and the corresponding drug delivery system must be strictly guaranteed to ensure that the side effects are within an acceptable range while the optimization effect is played. Currently, the safety and efficacy of COVID-19 vaccine liposomes are confirmed and recognized, which is encouraging news for the development of the PROTAC delivery system. At the same time, the PROTAC delivery system is designed to benefit a range of other chimeras with protein-degrading capabilities. Examples include LYTAC (a chimera targeting lysosomes) and ATTEC (autophagosome-tethering compound). Therefore, the exploration and evaluation of the PROTAC delivery system play a bridging role in the development of the whole field of protein degraders.
References
- Wang, Y., et al. Targeted degradation of anaplastic lymphoma kinase by gold nanoparticle-based multi-headed proteolysis targeting chimeras, Colloids and Surfaces B: Biointerfaces, 2020, 188, 110795.
- Zhang, C., et al. Semiconducting polymer nano-PROTACs for activatable photo-immunometabolic cancer therapy, Nature Communications, 2021, 12, 2934.
- Luo, G., et al. Discovery of an orally active VHL-recruiting PROTAC that achieves robust HMGCR degradation and potent hypolipidemic activity in vivo, Acta Pharmaceutica Sinica B, 2021, 11, 1300-1314.

 Fig 1. The depressant nanoparticles Cer/Pom-PEG@GNP1
Fig 1. The depressant nanoparticles Cer/Pom-PEG@GNP1 Fig 2. The combination therapy of PROTACs, phototherapy, and immunotherapy mediated by polymerized nanoparticles2
Fig 2. The combination therapy of PROTACs, phototherapy, and immunotherapy mediated by polymerized nanoparticles2 Fig 3. The structure of HMGCR-targeting PROTAC3
Fig 3. The structure of HMGCR-targeting PROTAC3