Pomalidomide derivatives play a central role in modern PROTAC development as reliable cereblon-recruiting ligands that enable efficient targeted protein degradation. As research programs move toward increasingly complex molecular designs, the synthetic route, structural integrity, and purity of pomalidomide intermediates have become critical determinants of experimental success. From scalable synthesis strategies and post-functionalization at key positions to advanced analytical testing and GMP-like production standards, controlling the quality of each derivative directly influences biological performance, reproducibility, and overall project progression. This article provides a comprehensive overview of the most widely adopted synthetic approaches, functionalization methods, and quality control practices, helping researchers build a solid foundation for high-confidence PROTAC discovery.
Introduction — Why Synthesis and Purity Are Crucial
Pomalidomide-based ligands have become an essential structural component in the design of next-generation PROTACs, thanks to their reliable E3 ligase recruitment and well-established pharmacological profile. Yet, the true performance of any pomalidomide derivative—whether used as a cereblon binder, a linker-bearing intermediate, or a final PROTAC warhead—ultimately depends on the precision of its synthetic route and the stringency of its purity control.
In modern targeted-protein degradation workflows, chemical integrity is not just a quality attribute; it is a functional determinant. Subtle differences in stereochemistry, residual impurities, or trace by-products can meaningfully alter degradation efficiency, binding affinity, and downstream biological interpretation. For research teams working under aggressive timelines, reproducibility and batch-to-batch consistency are therefore essential.
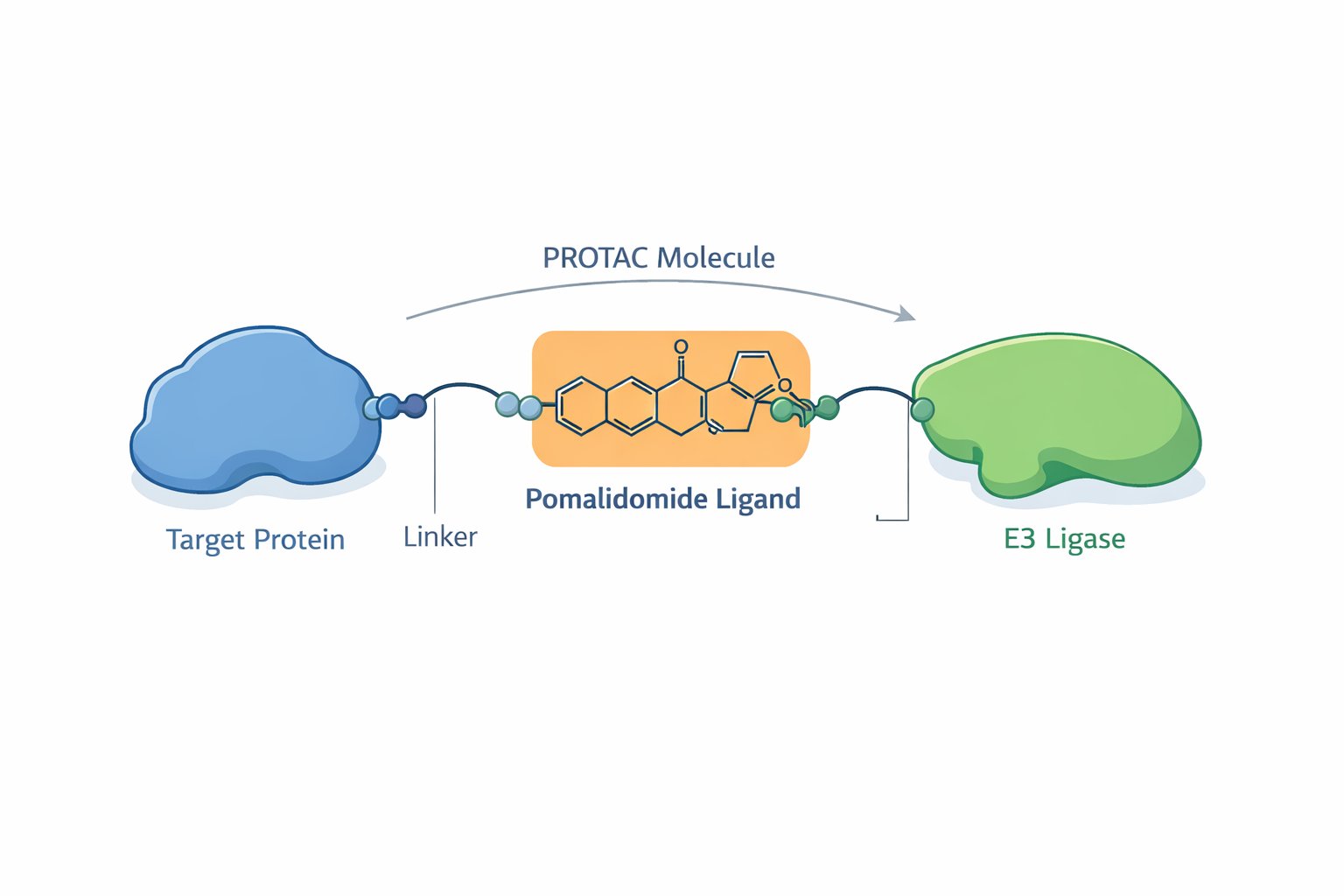 Conceptual illustration of a PROTAC molecule in which a pomalidomide-derived ligand recruits an E3 ligase to a target protein, enabling proximity-induced protein degradation.
Conceptual illustration of a PROTAC molecule in which a pomalidomide-derived ligand recruits an E3 ligase to a target protein, enabling proximity-induced protein degradation.
The Importance of Chemical Integrity in PROTAC Efficiency
A PROTAC molecule relies on orchestrating multiple molecular interactions simultaneously—target engagement, E3 ligase binding, and ternary complex formation. For pomalidomide derivatives, any deviation from the intended chemical structure can reduce cereblon-binding potency or modify the conformational landscape required for efficient ternary complex assembly. High structural fidelity ensures:
- Reliable biological activity across experiments
- Predictable degradation kinetics
- Accurate SAR conclusions during lead optimization
With development decisions increasingly driven by subtle SAR patterns, uncompromised quality in the pomalidomide fragment becomes a foundational requirement.
Impurities and Their Impact on Biological Results
Trace impurities may appear inconsequential from a synthetic perspective but can dramatically influence biological outcomes. Undesired isomers, partially modified intermediates, or residual reagents may:
- Compete with the active compound for binding sites
- Introduce off-target effects that obscure true degradation profiles
- Skew potency assessments, leading to misleading structure-activity relationships
Such complications not only waste resources but can derail project timelines. Robust purity control—supported by validated analytical methods—is therefore indispensable for ensuring that biological readouts reflect the true performance of the intended molecule.
Common Synthetic Strategies for Pomalidomide
The synthesis of pomalidomide derivatives has evolved significantly as demand for cereblon-recruiting ligands has increased within the PROTAC field. Although the overall scaffold is well established, research teams often require tailored variants—distinct substitution patterns, linker handles, or physicochemical modifications—that demand carefully selected synthetic routes. The overarching goal is to balance yield, selectivity, scalability, and compatibility with downstream functionalization steps.
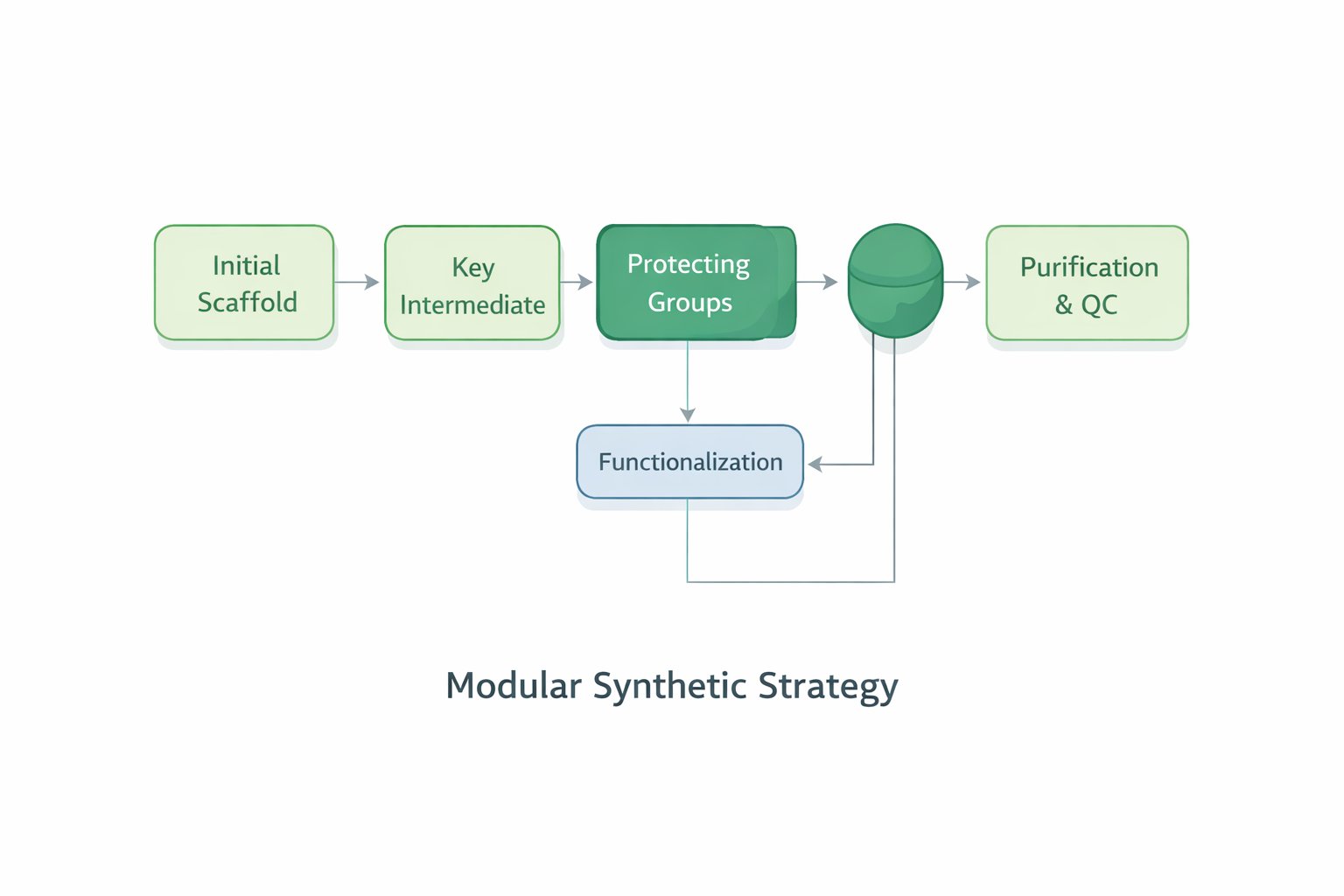 Modular synthetic strategy for the preparation and functionalization of pomalidomide-based ligands, illustrating scaffold construction, intermediate optimization, protecting group management, and final purification with quality control.
Modular synthetic strategy for the preparation and functionalization of pomalidomide-based ligands, illustrating scaffold construction, intermediate optimization, protecting group management, and final purification with quality control.
Stepwise Condensation and Cyclization Approaches
Most synthetic strategies begin with forming the glutarimide core followed by construction of the phthalimide moiety, typically achieved through controlled condensation and ring-closure steps. These modular approaches allow chemists to introduce structural diversity at defined positions without compromising the integrity of the cereblon-binding pharmacophore. For PROTAC development, the stepwise methodology is particularly advantageous because it enables:
- Precise positional substitution (e.g., C4 or C5 functionality)
- Fine-tuning of steric and electronic properties prior to linker installation
- Access to multiple analog series for rapid SAR exploration
By maintaining robust control over early intermediates, researchers ensure that each subsequent transformation proceeds from a highly reproducible foundation.
Protecting Group Selection for Optimal Yield
The choice of protecting groups—while often understated—plays a pivotal role in the efficiency of pomalidomide synthesis. Because the scaffold contains multiple reactive heterocycles, functional groups must be masked selectively to prevent undesired side reactions. Effective protecting-group strategies help researchers:
- Improve overall yields by minimizing decomposition pathways
- Enable orthogonal deprotection sequences for complex derivatives
- Preserve sensitive functionalities during harsher reaction conditions
This strategic orchestration is especially relevant when preparing linker-ready pomalidomide analogs for bifunctional molecule construction.
Green and Scalable Synthetic Variants
As PROTAC programs transition from exploratory research into early development, sustainability and scalability become important considerations. Modern synthetic variants increasingly emphasize:
- Reduced reliance on hazardous solvents
- Streamlined reaction sequences with fewer purification steps
- Cost-efficient, high-throughput production suitable for gram- to multi-gram scale
Adopting greener methodologies not only supports environmental responsibility but also strengthens supply-chain reliability—critical for organizations advancing multiple cereblon-based PROTAC candidates in parallel.
Post-Synthetic Modifications and Functionalization
Once the core pomalidomide scaffold is assembled, much of its value in PROTAC applications comes from the strategic modifications introduced afterward. These post-synthetic steps determine how effectively the molecule integrates into bifunctional degradation platforms, influences solubility or permeability, and supports fine-tuned target engagement. As a result, functionalization has become a central design consideration rather than a simple downstream step.
Table 1. Common Modification Sites of Pomalidomide and Their Applications
| Modification Site | Typical Functionalization Purpose | Impact on PROTAC Design |
| C4 Position | Installation of linear or PEG-based linker handles | Influences linker orientation and ternary complex geometry |
| C5 Position | Introduction of rigid or aromatic substituents | Enhances spatial alignment and degradation selectivity |
| Phthalimide Ring | Core cereblon-binding pharmacophore | Structural integrity is critical for CRBN engagement |
| Glutarimide Moiety | Maintains key hydrogen-bond interactions | Modifications may significantly reduce binding affinity |
Installing Linker Handle Groups (C4, C5)
Introducing substituents at the C4 or C5 position of the phthalimide ring is one of the most common strategies for preparing pomalidomide-based E3 ligase ligands. These positions offer accessibility for selective derivatization without disrupting cereblon binding. Appropriate installation of linker handles enables:
- Efficient attachment of alkyl, PEG-based, or aromatic linkers used in PROTAC design
- Modulation of linker orientation, which directly affects ternary complex formation
- Optimization of degradation selectivity through spatial control of protein-protein interfaces
Because even slight changes in linker geometry can dramatically influence degradation efficiency, reproducibility and precision in these modifications are crucial.
Derivatization for Increased Solubility or Stability
Beyond linker attachment, pomalidomide derivatives can be further modified to enhance physicochemical and biopharmaceutical properties. Thoughtfully designed substituents may:
- Improve aqueous solubility, supporting more accurate in vitro assessments
- Increase metabolic stability, extending the half-life of PROTAC candidates
- Enhance permeability, critical for cellular assays and early ADME profiling
- Reduce undesired aggregation, enabling more consistent degradation readouts
Such modifications provide researchers with significant control over how the pomalidomide fragment behaves within a larger multifunctional molecule, ultimately helping accelerate program advancement from screening to lead optimization.
Analytical Purity and Quality Control Methods
High-quality pomalidomide derivatives are foundational to reliable PROTAC research, and rigorous analytical characterization is essential for ensuring structural accuracy, purity, and long-term stability. Since even trace impurities can influence degradation efficiency, each batch must undergo systematic quality evaluation supported by validated analytical platforms.
Table 2. Analytical Methods Used for Pomalidomide Purity and Identity Verification
| Analytical Method | Primary Purpose | Information Obtained |
| HPLC | Quantitative purity assessment | Purity percentage and impurity profile |
| LC-MS | Molecular weight confirmation | Accurate mass and structural consistency |
| NMR (1H / 13C) | Structural and substitution verification | Chemical framework and functional group placement |
| Elemental Analysis | Composition validation | Agreement with theoretical elemental values |
| Stability Testing | Storage and shelf-life evaluation | Degradation trends and optimal storage conditions |
HPLC and LC-MS for Structural Validation
High-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS) are the primary tools for verifying compound purity and identity. These methods allow researchers to:
- Quantify purity with high sensitivity, detecting low-level impurities undetectable by conventional techniques
- Confirm molecular mass and fragmentation patterns, ensuring the correct pomalidomide derivative is present
- Monitor batch consistency, which is crucial for reproducible biological studies
For pomalidomide intermediates with linker handles or modified phthalimide groups, LC-MS becomes particularly valuable for confirming successful functionalization steps.
NMR and Elemental Analysis for Identity Confirmation
NMR spectroscopy provides detailed structural information, enabling confirmation of substitution patterns, ring integrity, and stereochemical configuration. Combined with elemental analysis, it offers:
- Definitive verification of the molecular framework
- Insight into potential tautomeric or conformational variations
- Confidence in the absence of residual solvents or unexpected heteroatoms
These techniques reinforce the chemical integrity required for downstream PROTAC development.
Stability Testing Under Storage Conditions
Because pomalidomide derivatives may be sensitive to moisture, light, or thermal fluctuations, stability assessment is an essential component of quality control. Stability studies help determine:
- Optimal storage conditions and packaging materials
- Shelf-life under standard laboratory environments
- Degradation pathways that may inform formulation or handling practices
Well-characterized stability profiles allow research teams to depend on consistent performance across long-term experimental timelines.
Our Manufacturing Capabilities and Quality Standards
As the demand for cereblon-binding ligands and custom pomalidomide derivatives accelerates within the PROTAC landscape, reliable manufacturing becomes just as important as scientific innovation. Our production capabilities are designed to meet the needs of discovery teams, process chemists, and translational researchers who require consistent quality, transparent documentation, and dependable supply chains.
GMP-Like Production and Batch Traceability
Although research-grade materials do not always require full GMP certification, our manufacturing workflow adheres to GMP-like principles to ensure exceptional quality and repeatability. Every batch is produced within a controlled, audit-ready environment, supported by:
- Comprehensive batch records and traceable starting materials
- Qualified equipment and standardized operating procedures
- In-process controls that maintain consistency across production runs
This approach reduces variability and provides researchers with greater confidence in long-term program reproducibility.
Customized Purity Specifications per Research Need
Different project stages demand different purity levels. Early screening may accommodate flexible specifications, while preclinical candidate development typically requires tighter controls. We offer:
- Adjustable purity grades, from exploratory-level to high-purity, near-pharmaceutical standards
- Tailored impurity profiles, including limits for related substances and solvent residues
- Analytical packages aligned to customer SOPs or regulatory expectations
By aligning purity specifications with the intended application, teams can optimize cost-efficiency without compromising scientific accuracy.
Advantage: Reliable Supply, Full Documentation, and Regulatory Readiness
Our customers benefit not only from robust chemistry but from a supply chain designed to support sustained research momentum. Key advantages include:
- Reliable, scalable production capacity for both standard and custom pomalidomide derivatives
- Full analytical documentation, including COAs, method descriptions, and stability notes
- Regulatory-ready data packages to facilitate future IND-enabling studies
- Dedicated technical support, ensuring smooth onboarding and troubleshooting
Whether your program focuses on hit validation or lead optimization, our quality-driven approach helps de-risk critical research steps.
Accelerate Your PROTAC Research With High-Purity Pomalidomide Derivatives
As PROTAC development becomes increasingly competitive, access to reliable, well-characterized pomalidomide derivatives can be the difference between clear, actionable data and stalled project timelines. Our team supports every stage of your discovery pipeline with precision synthesis, stringent analytical validation, and fully traceable documentation. Whether you're evaluating new cereblon-recruiting ligands, optimizing linker design, or preparing materials for downstream studies, we provide the quality and technical support needed to move your research forward with confidence.

 Conceptual illustration of a PROTAC molecule in which a pomalidomide-derived ligand recruits an E3 ligase to a target protein, enabling proximity-induced protein degradation.
Conceptual illustration of a PROTAC molecule in which a pomalidomide-derived ligand recruits an E3 ligase to a target protein, enabling proximity-induced protein degradation. Modular synthetic strategy for the preparation and functionalization of pomalidomide-based ligands, illustrating scaffold construction, intermediate optimization, protecting group management, and final purification with quality control.
Modular synthetic strategy for the preparation and functionalization of pomalidomide-based ligands, illustrating scaffold construction, intermediate optimization, protecting group management, and final purification with quality control.