Protacs and its related targeted degradation techniques (such as LYTACs, dTAGs, Trim-Away and SNIPERs) have become new therapeutic methods for targeting proteins that are traditionally considered unavailable. However, because the ubiquitin-mediated degradation pathway occurs in the cell, the small molecule Protacs mainly attacks the intracellular targets, which makes many membrane proteins (accounting for 23% of the encoded proteins) can not be targeted by Protacs technology, because these proteins have no suitable binding pockets for small molecular ligands to bind in the intracellular segment. Therefore, a new strategy for the degradation of cell surface proteins may revolutionize the field of targeted protein degradation.
In a recent study published in the journal Journal of the American Chemical Society, a team of scientists from the University of California, San Francisco suggested that antibody-based Protacs (AbTACs) may be a direction worth exploring. Their study reported a fully recombinant bispecific antibody that can recruit membrane-bound E3 ligases to degrade cell surface proteins. Studies have shown that AbTACs can degrade PD-L1 through recruitment membrane binding to E3 ligase RNF43.
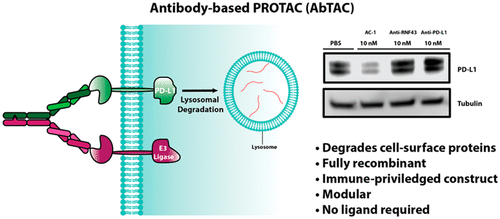
RNF43 is a transmembrane E3 ligase, including an extracellular domain with a specific structure and an intracellular RING domain. RNF43 can induce endocytosis and degradation by ubiquitination of Wnt coreceptor Frizzled, and then negatively regulate Wnt signaling pathway (previously developed small molecules of Protacs use cytoplasmic E3 ligase, this study expands the application of transmembrane E3 ligase in targeted degradation).
In the study, scientists chose PD-L1 as the initial degradation target. PD-L1 is a protein overexpressed in many cancers, which results in the inhibition of T cell response by binding to the inhibitory receptor PD-1 on T cells. PD-L1 has a small cytoplasmic domain of 31 amino acids, but no known small molecular ligands that can bind to this domain, which makes it challenging to target PD-L1 with traditional small molecules of Protacs.
In order to construct PD-L1 targeting AbTACs, PD-L1 antibody atezolizumab was used as PD-L1 binder, and fused with RNF43 binder to produce recombinant bispecific antibody AC-1. BLI test confirmed that AC-1 can combine PD-L1 and RNF43 at the same time. Next, the researchers tried to test whether AC-1 could degrade PD-L1 on cells. Because of the high expression of PD-L1, they first chose the triple negative breast cancer cell line MDA-MB-231. The presence of RNF43 in MDA-MB-231 was confirmed by flow cytometry. The results showed that MDA-MB-231 cells treated with 10nM AC-1 could induce a large number of degradation of two forms of PD-L1 within 12h, and reached the highest level (Dmax=63%) at 24h. To test whether AC-1’s degradation of PD-L1 depends on RNF43, the researchers knocked out the RNF43 in MDA-MB-231 cells using CRISPR interference (CRISPRi). The results showed that after being treated with 10nM AC-1 for 24h, this AbTACs did not induce the degradation of PD-L1 in these RNF43 knockout cells. In order to evaluate the degradation pathway, pretreatment was carried out with bafilomycin (a lysosomal acidification inhibitor) or MG-132 (a proteasome inhibitor). The results showed that bafilomycin could reduce the degradation of PD-L1, but MG-132 could not. These data indicate that AC-1 induces PD-L1 degradation in a RNF43-and lysosomal-dependent manner.
Finally, the scientists tested whether AC-1 could degrade PD-L1, in other cell lines, including triple negative breast cancer cell line MDA-MB-231, non-small cell lung cancer cell line HCC827 and advanced bladder cancer cell line T24. The results showed that PD-L1 degradation could be induced by 10nM AC-1 treatment for 24h in each cell line, indicating that the antibody-based Protacs technique had wide cellular applicability.
The researchers believe that AbTACs represents a new protein degradation agent technology, and it is worth further exploring its potential to degrade membrane proteins. In this paper, they also focus on another new protein degradation technology, LYTACs, which depends on lysosome degradation pathway and can be used to degrade membrane proteins.
LYTACs, whose full name is lysosome-targeting chimaeras, or lysosome targeted chimera, was developed by scientists at Stanford University. LYTACs consists of an oligoglycopeptide group, an antibody and a linker. Oligosaccharide peptides bind to a cell surface receptor called CI-M6PR, a lysosome-targeted receptor, and antibodies bind to a specific transmembrane or extracellular protein. When LYTAC binds to CI-M6PR and target protein at the same time, the resulting CI-M6PR-LYTAC- target protein complex will be "swallowed" by the cell membrane to form a transport vesicle. The vesicles transport the complex to the lysosome (an organelle containing protein-degrading enzymes), where the target protein is degraded and the receptor is recycled. This important finding was published last July in the journal Nature. In this study, scientists also successfully used LYTACs to degrade PD-L1. Scientists believe that LYTACs as an effective tool to expand the range of degradable proteins, the prospect is very promising.
As stated in a review article in Nature magazine, with the maturity of existing protein degradation technologies and the emergence of new protein degradation technologies, it is possible that one day all proteins can be targeted for degradation. By then, there may be a large number of milestones in the field of disease treatment.
References
- Cotton, A. D., Nguyen, D. P., Gramespacher, J. A., Seiple, I. B., & Wells, J. A. (2021). Development of antibody-based Protacs for the degradation of the cell-surface immune checkpoint protein PD-L1. Journal of the American Chemical Society, 143(2), 593-598.
- Banik, S. M., Pedram, K., Wisnovsky, S., Ahn, G., Riley, N. M., & Bertozzi, C. R. (2020). Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature, 584(7820), 291-297.


