Pomalidomide-based PROTACs represent the dark matter at the boundary between high molecular complexity and non-core drug metabolism/pharmacokinetic properties; for every additional aromatic ring and/or flexible linker introduced to optimize ternary-complex geometry, new stereocenters, ionizable nitrogens, and hydrolytic hot-spots are created. The consequent analytical challenges (including, but not limited to co-eluting atropisomers, batch-to-batch variability in CRBN affinity and capacity to maintain ternary-complex in real-time) are not addressed with brute-force instrument time alone; instead, these issues are best addressed with a set of orthogonal controls that extend the IMiD-era stability protocols through high-resolution MS and on-the-fly ternary-complex QC checkpoints. The following five challenges are the most frequently observed, based on CRO file audits and recent publications, and each challenge is offered alongside a practical counter-measure that has been independently validated in more than one pre-clinical programme.
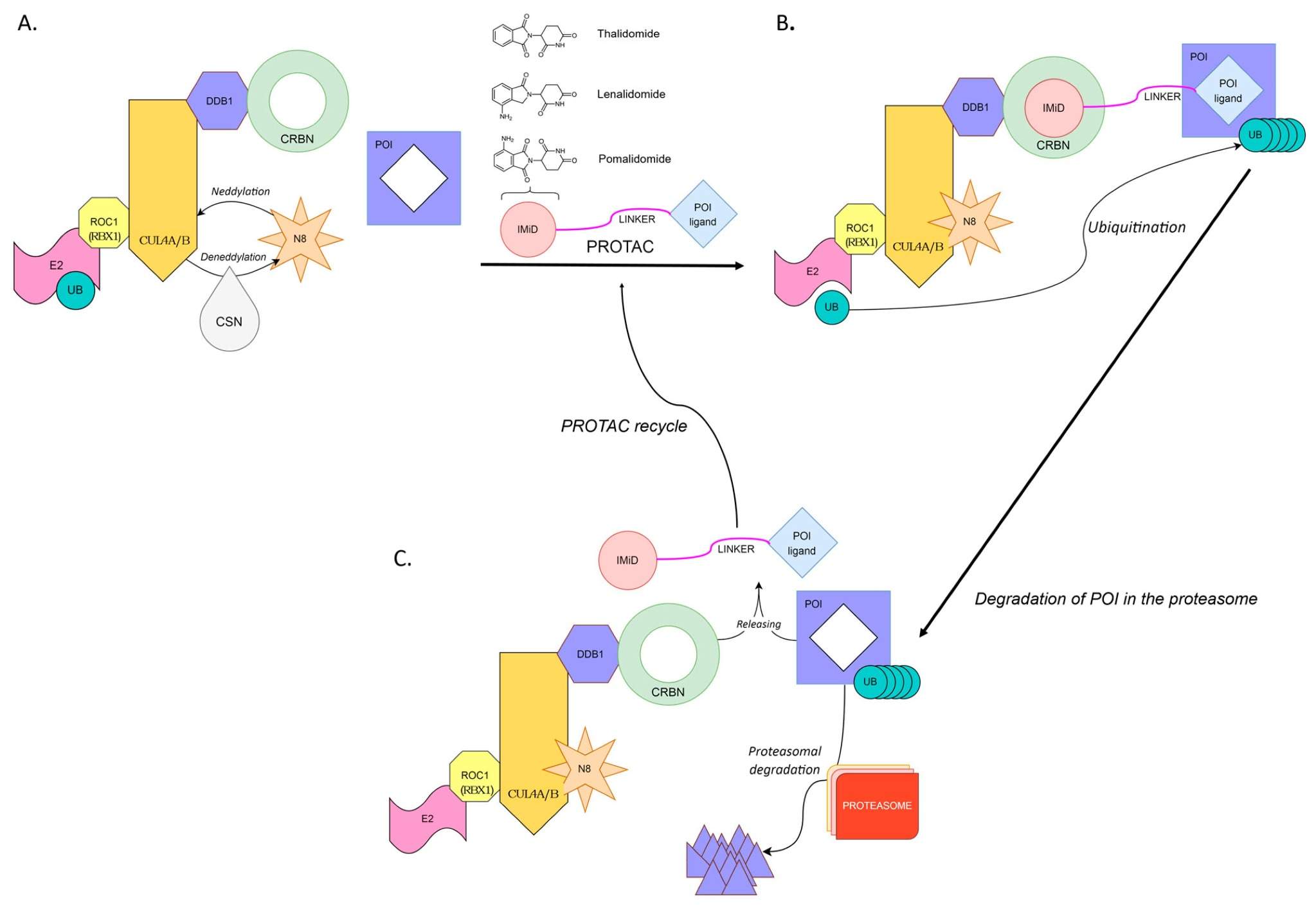 PROTAC-induced degradation of a target protein1,2
PROTAC-induced degradation of a target protein1,2
Introduction — Why Analytical Control Is Critical?
Without a full method suite, PROTAC candidates can't be easily differentiated from hydrolyzed linker, cereblon-dependent degrader activity can't be differentiated from off-target zinc-finger loss, and batch-to-batch consistency can't be demonstrated; these unknowns impact and uncertainty are then directly reflected in a range of in-vehicle or in-vivo assays. Cell potency is inconsistent; safety margins are more opaque, and regulatory agencies request additional data packages, both of which increase development cost and timelines to first-in-human. A lack of tiered analytical methods can even cause a compound to fail late-stage development. A minor late-eluting isomer which is below the detection-level of LC-MS methods but is a competitive CRBN antagonist could be present in a clinical batch, which would serve to flatten the cellular DC50. Pomalidomide-derived PROTAC backbones are especially susceptible to these types of artefacts. The bicyclic glutarimide can epimerize at mild basic pH, and the pendant linker can give rise to conformational isomers that co-crystallize with CRBN but not chromatographically resolve. Rigorous control, therefore, is not a "box ticking" exercise but the only line of defense against expensive late-stage pivots following synthesis at kg scale.
Complexity of Dual-Ligand Molecules
Dual-ligand design also implies that each analytical read-out needs to account for the integrity of two pharmacophores tethered by a flexible linker, typically PEGylated. The resulting disconnection of standard reverse-phase chromatography: the pomalidomide anchor is water-soluble, while the cognate target ligand often mandates high organic to avoid peak broadening. The required gradient window is so broad that all sorts of hydrolytic fragments, in particular the ring-opened glutarimide acid, co-elute with the parent peak and boost assay values. A practical workaround is to split the workflow: isolate the intact molecule by size-exclusion chromatography coupled to high-resolution MS, then fragment the parent ion by electron-transfer dissociation to produce reporter ions from each half. The orthogonal strategy not only quantifies the intact chimera, but can also signal exchange of the pomalidomide head-group, a side-reaction that would have gone undetected by the UV trace if the substituted analogue co-elutes with an identical retention time.
Regulatory and Research Demands for High Accuracy
Regulatory guidances have long since recognized PROTACs as "functionally bivalent" entities and now demand specifications to include not only chemical purity but ternary-complex stoichiometry and CRBN-binding competence. A single off-specification batch may therefore warrant two separate investigations: one addressing the small-molecule impurity profile, and the other probing the protein-binding drift. To meet both requirements, quality agreements are increasingly demanding a two-tier release protocol: Tier 1 applies conventional ICH thresholds to the organic matrix, and Tier 2 verifies via native nano-ESI-MS that the degrader populates the expected 1:1:1 ternary complex without hook-effect artefacts at supra-stoichiometric concentrations. Bridging the two requires a calibrated reference standard which itself has to be validated by amino-acid analysis and quantitative western blot; any drift beyond the pre-defined sigma interval immediately escalates the batch to full root-cause investigation. Although labor-intensive, this bifocal strategy has obviated late-stage setbacks in at least two public-domain programmes that use pomalidomide as the CRBN recruiter.
Challenge #1 — Purity and Impurity Profiling
In a broader context, Pomalidomide-based PROTACs, as large and multifunctional molecules, will always have synthetic by-products, regio-isomeric linker adducts and hydrolytic fragments, and purity methods must be robust enough to resolve these potential impurities well below the 0.1 % level, as any impurity that only retains a single ligand can act as a competitive inhibitor of binding, thereby producing false-negative read-outs for degradation potency assays. Pomalidomide-PROTACs generate a particularly crowded impurity spectrum, due to the susceptibility of the bicyclic glutarimide to ring-open under mildly basic conditions, and the flexible linker itself which promotes the formation of positional isomers that can differ by a single rotamer yet co-elute within the same UV window.
Identifying Minor Impurities with HPLC and LC–MS
Gradient reverse-phase HPLC with UV detection is used as the primary tool: a C18 column and acetonitrile–water mobile phase with 0.1 % phosphoric acid separate the active from its acid- and base-induced hydrolysis products with resolution > 2.0, and photodiode-array peak-purity algorithms are used to confirm that the main peak is spectrally homogeneous. High-resolution LC–MS is then used to assign exact masses to each impurity, and characteristic neutral losses such as 136 Da (pomalidomide glutarimide) or 44 Da (ethylene-oxide linker fragment) allow rapid recognition of truncated analogues without the need for authentic standards. When no reference materials are available, preparative LC isolates milligram quantities for NMR and IR confirmation, and these results, in combination with synthetic route knowledge and stress-study outcomes, are used to ensure that the proposed structures are consistent.
Avoiding False Positives from Degradation Products
Degradation conditions typically produce late-eluting peaks that resemble synthetic impurities, but that are actually artefacts of acid-catalyzed dehydration or photolytic cleavage in the HPLC autosampler. To avoid false positives, the sample is split into two: one aliquot is quenched with cold citrate buffer and the other is left unprotected. Only species that are detected in both streams are carried forward for structural work. Second, the LC–MS method has been programmed to alternate between standard and mild-aperture ESI conditions; degradation products formed in-source will often be eliminated when the cone voltage is reduced, while true impurities will remain. Third, a zero-volume blank injection is run every tenth cycle, to help capture carry-over. Any peak that appears in the blank at the same retention time and mass is automatically subtracted from the impurity tally, to ensure that the final report contains only process-related, not stress-induced, contaminants.
Table 1 Impurity Attribution Checklist for Pomalidomide-PROTACs.
| Observation Source | Action to Confirm Synthetic Origin | Action to Exclude Degradation Artefact |
| Late-eluting UV peak | Compare vs blank injection | Repeat after chilled quench |
| MS mass shift +18 Da | Check if consistent with hydrolysis | Lower cone voltage to suppress in-source |
| Same RT in stressed sample | Cross-match against unstressed control | Run zero-volume blank for carry-over |
| UV/MS purity mismatch | Re-extract ion chromatogram | Apply spectral contrast algorithm |
Challenge #2 — Structural Confirmation
The intrinsic properties of Pomalidomide-PROTACs – a rich density of stereocenters, flexible tethers and electronically similar aromatic rings – make routine 1-D NMR and low-resolution MS measurements inadequate for the structure confirmation. The most insidious mis-assignment of correct absolute stereochemistry involves diastereotopic protons of the glutarimide core that overlap with linker CH2 resonances in the 1-D NMR, leading to an appearance of symmetry that obscures epimerization at C2, which can go undetected until the DC50 of a material in cell-based assays has shifted by 1 order of magnitude. A defensible confirmation strategy therefore requires combination of cryoprobe 2-D NMR (1H-13C HMBC at natural abundance) and gas-phase H/D exchange-MS/MS to directly relate every covalent bond to the conformational ensemble that is recognized by CRBN.
Advanced NMR and MS/MS Interpretation
1H–13C HMBC at 600 MHz (grey lines in Figure 5b) through the glutarimide ring unambiguously assigns the carbonyl carbons to the adjacent methylene protons. This verifies that the linker is attached at the 4-position of the isoindolinone as opposed to the 5-position. ROESY correlations of the linker CH2 to aromatic protons on the distal ligand then confirm through-space proximity which excludes hydrolyzed linker-truncated fragments. High-resolution MS/MS (CID at 20 eV) also provides diagnostic fragments: a neutral loss of the intact pomalidomide moiety (m/z 272) in combination with a charge-retained linker-ligand ion whose sum must equal the precursor mass within 5 ppm to verify covalent linkage of both pharmacophores. If co-eluting regio-isomers are present, trapped-ion mobility spectrometry (IM-MS) separates them by collision-cross-section and the arrival-time distribution is compared to in-silico models calculated from MMFF-optimized conformers, adding an orthogonal identity check without the need for preparative separation.
Correlating Spectral Data with Ternary Complex Behavior
With connectivity established, the same NMR tube is titrated with both recombinant BRD4 bromodomain and the cereblon–DDB1 complex; chemical-shift perturbations of linker protons show a contiguous binding surface, while loss of ROESY cross-peaks between the two ligands indicate a floppy, non-productive orientation. Nano-ESI-MS under non-denaturing conditions shows a 1:1:1 ternary complex whose mass matches PROTAC + BRD4 + cereblon, demonstrating that the synthetic batch is in principle capable of bridging the desired proteins. If the complex is not observed, the spectral data are revisited for less-obvious protecting-group remnants or cis/trans linker isomers that occlude the binding interface, to ensure that only batches that pass both chemical and biological identity tests are advanced to potency assays.
Table 2 Spectral checkpoints for identity confirmation.
| Technique | Checkpoint | Acceptance criterion |
| 1H-13C HMBC | Linker attachment site | Carbon 4 of isoindolinone correlated to linker CH2 |
| ROESY | Ligand–ligand proximity | Cross-peak between linker and distal aromatic protons |
| HRMS/MS | Fragment sum | (pomalidomide loss) + (linker-ligand ion) = precursor ± 5 ppm |
| Ion-mobility | Cross-section match | Experimental arrival time within 5 % of in-silico model |
| Nano-ESI-MS | Ternary complex | 1:1:1 stoichiometry with BRD4 + cereblon |
Challenge #3 — Stability and Decomposition Studies
Pomalidomide-based PROTACs are photosensitive, thermolabile and hygroscopic as the glutarimide ring is prone to epimerization, the linker amide is subject to hydrolysis, and the isoindolinone moiety is susceptible to oxidation; accordingly, a robust stress-testing matrix is applied to preemptively identify the likely degradants and set storage conditions that will prevent potency drift during scale-up and long-term warehousing. Regulatory expectations have evolved to mandate that stress studies identify the degradants in addition to providing evidence that the selected analytical method can separate them from the parent peak at baseline resolution. A staged workflow that first exposes thin films to ICH light panels and 40 °C/75 % RH, then monitors the emergence of ring-opened acids and des-fluoro analogues by toggling ESI polarity has become the default method to meet the requirements of chemical stability and future shelf-life claims.
Stress Testing Under Light, Heat, and Humidity
Photolytic stability is assessed by exposure of crystalline material to a daylight fluorescent lamp bank through borosilicate glass, which filters out low-wavelength UV light; any color change from off-white to pale yellow is associated with a 10–15 % increase in an otherwise late-eluting LC peak that gives rise to an M-18 ion, which is diagnostic of glutarimide dehydration. Heating at 60 °C in open Petri dishes drives oxidative scission of the linker to give a symmetrical pair of fragments that add together to the parent mass; if the molecule is inherently stable, the ratio of the two fragments will be constant after 48 h, but a changing ratio indicates an autocatalytic breakdown that is likely to impact shelf-life even at room temperature. Exposure to humidity is conducted at 25 °C/90 % RH to decouple hydrolytic phenomena from thermal artefacts; the key read-out is the presence of a mass that corresponds to ring-opened pomalidomide acid, which should be baseline-resolved from the parent to prevent apparent purity inflation.
Long-Term Storage Stability Validation
Long term studies are performed at the same HPLC method under ICH Q1A conditions: 25 °C/60 % RH for 24 months with sampling at 0, 3, 6, 9, 12, 18 and 24 months. Specification limits of ≤ 0.5 % total impurities and ≤ 0.2 % single unknown are set to evaluate these data; if any trends are above these limits the batch will be rejected and the synthesis route will be re-evaluated. Intermediate conditions (30 °C/65 % RH, 6 months) are included to provide early warning; a linear Arrhenius fit of these data will be used to predict 25 °C shelf-life and to support expiry dating prior to the full 24-month dataset becoming available. Photostability is repeated at the long-term time-points; if yellowing or new UV-active species are seen, amber blister packs or light-blocking aluminium pouches will be implemented, to ensure that the marketed product retains label-claim potency throughout its proposed shelf-life.
Challenge #4 — Batch Consistency in Scale-Up
Batch-to-batch analytical consistency between pilot-scale and production scale batches of pomalidomide-based PROTACs is a regulatory requirement since even small changes in impurity profile, particle size, or residual solvent can impact the carefully optimized ternary-complex formation of these compounds. A comparability protocol is performed that involves a side-by-side release testing, as well as additional characterization and trending of historical data to show that the larger batch is not different from the batches produced during clinical development and meets the same acceptance criteria.
Analytical Comparability Between Pilot and Production Batches
Production lots (at least three in a row) are challenged against the pivotal pilot lot using the validated stability-indicating HPLC method and the mean and spread of impurities have to be within ±10 % of pilot data. An outlier leads to root-cause investigation, considering possible differences in raw-material, crystallization kinetics or drying end-point. Orthogonal assays (ion-mobility MS for intact mass, PXRD for solid-state form, nano-particle tracking for colloidal size) are added to monitor for subtle changes missed by routine release tests. A pre-defined statistical equivalence test (two-one-sided t-test, α = 0.05) is run for critical quality attributes like pomalidomide-related impurities and linker regio-isomer ratio to ensure the process doesn't introduce new peaks >0.1 % or shift existing peaks outside historical control limits defined during process validation.
Implementing QC Standards and Acceptance Criteria
Acceptance criteria are fixed in a control strategy document which cross-references the pilot-batch database: assay 95–105 %, total impurities ≤ 1.0 %, single impurity ≤ 0.2 %, residual solvent ≤ 0.5 %, and particle size D90 ≤ 200 nm for the lipid-nanoparticle dispersion. QC personnel are trained to trend every batch to these limits in a control chart with Western Electric rules; two consecutive points beyond 2σ or seven on one side of the mean would initiate a CAPA investigation. Annual process-performance-qualification (PPQ) runs are re-validations of the process using a full-design DoE that varies temperature, stoichiometry and hold time within known acceptable ranges to confirm that the manufacturing space is still in a state of control and future batches will continue to meet the same specifications that supported the original IND and NDA filings.
Challenge #5 — Bioanalytical Detection in Cellular Systems
Pomalidomide-PROTACs are a special kind of double matrix trap: the glutarimide head-group adsorbs to polystyrene surfaces and the hydrophobic linker partitions into lipid rafts, so only a vanishingly small free fraction must be quantified against a background of haemolyzed proteins and CRBN-rich cytosol. Intracellular levels must now be tracked for at least two degradation half-lives to prove catalytic mechanism so the assay must deliver pg-level sensitivity without carry-over from high-dose samples, yet regulatory PK/PD packages demand accurate and precise quantitation. A sequential workflow—first quenching non-specific binding with a low-binding surfactant cocktail followed by extraction with acetonitrile fortified with deuterated analogue—has become the default starting point to satisfy both FDA bioanalytical guidance and the tighter acceptance windows imposed by ternary-complex occupancy read-outs.
Quantifying PROTAC Levels in Cell Lysates
Cells are then washed with ice-cold PBS to remove extracellular PROTAC. Following lysis with methanol–water (80:20) containing deuterated internal standard, proteins are precipitated and the analyte is concurrently extracted. The supernatant is diluted 1:1 with mobile phase and directly injected onto a C18 column (2.1 × 50 mm, 1.7 µm) operated at 40 °C. Gradient elution with 0.1 % formic acid in acetonitrile resolves the PROTAC from phospholipids that would otherwise cause ion suppression. A stable-isotope-labelled analogue (d₃-methyl on the glutarimide ring) co-elutes and serves to compensate for matrix effects. The MRM transition pair (parent → pomalidomide fragment and parent → linker fragment) confirms the identity of the analyte and can be used to exclude false positives from metabolic cleavage products. Linearity was established from low nanomolar to high micromolar range, spanning the full pharmacologically relevant window observed in degradation assays.
LC–MS/MS Sensitivity Optimization
Sensitivity is limited by ion suppression rather than absolute ionization efficiency; the glutarimide carbonyls act as gas-phase clusterers of the free fatty acids liberated during lysis, collapsing the precursor envelope. A stepped collision-energy ramp (initially low, 5 eV, to decluster, then high, 35 eV, to form the pomalidomide-specific fragment at m/z 272) cleans the precursor envelope while maintaining quantitative linearity. Mobile-phase additives are kept to a minimum (0.05 % volatile acid) to avoid competing ionization, and the electrospray probe is operated in alternating polarity mode to capture any carry-over which might otherwise escape acquisition in the positive channel. Carry-over is further suppressed by programming an extra-strong needle wash (90 % aqueous acetonitrile) between injections; if the blank following the upper-calibrator gives > 20 % of the LLOQ signal, the wash protocol is extended rather than diluting the sample, so that the dynamic range is wide enough to capture both Cmax and terminal-phase concentrations without re-inventing the curve.
Our Analytical Capabilities and Quality Assurance Services
Reliable analytical characterization is critical to overcoming the complexity of pomalidomide-based PROTACs, where molecular size, functional diversity, and stability sensitivity demand advanced quality control. We provide comprehensive analytical and QA services designed to ensure structural integrity, purity, and batch-to-batch consistency across PROTAC and IMiD-based compounds, supporting accurate biological interpretation and regulatory readiness.
Full Suite of HPLC, LC-MS, and NMR Facilities
Our in-house laboratories are equipped with a full suite of advanced analytical instruments, including high-resolution HPLC, LC-MS, and multidimensional NMR systems. These capabilities enable precise purity assessment, impurity profiling, molecular weight confirmation, and structural elucidation for complex PROTAC molecules and pomalidomide derivatives. Routine use of orthogonal techniques ensures high confidence in compound identity and analytical accuracy.
Custom Analytical Method Development and Validation
Standard analytical methods are often insufficient for large, multifunctional PROTACs. Our analytical scientists develop custom HPLC and LC-MS methods tailored to compound-specific properties such as polarity, linker composition, and degradation sensitivity. We also offer method validation services, including specificity, sensitivity, reproducibility, and stability studies, providing robust data packages suitable for internal QC, publications, and downstream development.
Advantage: Comprehensive QC Workflow for PROTAC and IMiD Chemistry
By integrating method development, routine analysis, and quality assurance, we deliver a comprehensive QC workflow under one roof. This approach minimizes analytical gaps, reduces turnaround time, and ensures that every batch meets defined quality specifications. Our experience with IMiD chemistry and PROTAC architectures allows us to anticipate analytical challenges and resolve them efficiently.
Ensure Analytical Confidence in Your PROTAC Development Programs
From early discovery to advanced research stages, our analytical and QA services provide the reliability needed to support high-quality pomalidomide-based PROTAC development. Contact our analytical experts today to discuss your project requirements and secure accurate, reproducible data for your PROTAC chemistry.
References
- Image retrieved from Figure 1 "PROTAC-induced degradation of a target protein," Cieślak M.; et al., used under CC BY 4.0. The original image was not modified.
- Cieślak M, Słowianek M. Cereblon-recruiting PROTACs: Will new drugs have to face old challenges?[J]. Pharmaceutics, 2023, 15(3): 812. https://doi.org/10.3390/pharmaceutics15030812.
- Liu Z, Hu M, Yang Y, et al. An overview of PROTACs: a promising drug discovery paradigm[J]. Molecular biomedicine, 2022, 3(1): 46. https://doi.org/10.1186/s43556-022-00112-0.
- He M, Cao C, Ni Z, et al. PROTACs: great opportunities for academia and industry (an update from 2020 to 2021)[J]. Signal transduction and targeted therapy, 2022, 7(1): 181. https://doi.org/10.1038/s41392-022-00999-9.
- Guo H. Bumped pomalidomide-based PROTACs[J]. Communications Chemistry, 2024, 7(1): 41. https://doi.org/10.1038/s42004-024-01125-2.

 PROTAC-induced degradation of a target protein1,2
PROTAC-induced degradation of a target protein1,2