Aromatic linkers, once considered to be run-of-the-mill synthetic intermediates, have emerged as mission-critical ingredients, where batch-to-batch consistency can mean the difference between an effective degradation campaign, or one that fails without obvious reason. While high quality lots come at a premium, they are differentiated not only by their higher levels of purity, but also by their thermal robustness, controlled trace-metal status, and pre-validated shipping conditions that allow scientists to run assays within hours of their arrival. Recent reports indicate that even at sub-percent levels, oxidized side-products can nucleate aggregation, resulting in false-negative read-outs in cell-based assays; on the other hand, linker lots that survive accelerated heating, without color change or hydrolytic cleavage, translate more smoothly from biochemical titration to in vivo studies. The sections below will go into more detail about the technical aspects that differentiate research-grade linkers from commodity material, and why storage stability is essential for reproducible PROTAC research.
Introduction to Premium Aromatic Linkers
Essentially, "premium" means that the specification envelope is tighter than usual thanks to iterative stress testing, in-process analytics and container-closure compatibility testing. A premium aromatic linker, for example, comes with a proven track record of resistance to hydrolytic, oxidative and photochemical degradation that's been confirmed using International Council for Harmonization (ICH)-aligned conditions but with results communicated in academic group-accessible language. Furthermore, these lots are designed for direct dissolution without recrystallisation, which in turn minimizes the chances of solvent or silicone oligomers that linger in the septa from contaminating sensitive biochemical assays. In short, the linker is delivered as a reagent, not a synthetic oddity.
What Makes a Linker "Premium"?
Promotion of an ordinary linker to "premium" status is less a single Eureka moment than the sum of small improvements in every stage of the molecule's life-cycle. Design begins with an aromatic core chosen so that the π-system is sufficiently conjugated to withstand oxidative cleavage, yet not so extensive as to permit unwanted π–π stacking interactions that destroy solubility. Synthetic access is the next hurdle: routes that circumvent heavy-metal catalysts or high-temperature nitration minimize the risk of trace-metal contaminants that poison downstream catalytic steps. Purification is where many molecules fall at the first hurdle. A premium batch will typically be put through a series of orthogonal separations, including adsorptive filtration to remove polar dyes, recrystallisation to reject constitutional isomers, and finally sublimation or preparative sub-critical fluid chromatography to purge residual solvents whose proton signals would otherwise intrude in NMR spectra. Characterization follows a similar belt-and-braces philosophy: infra-red assignment of diagnostic out-of-plane bending modes, high-resolution mass measurement that confirms the elemental composition to within 2 ppm, and quantitative 13C NMR to expose even sub-percent levels of regioisomeric impurities. Once the solid is spectroscopically clean, stability testing is initiated. Accelerated ageing for 8 weeks at 40 °C under 75 % relative humidity is customary; premium lots show<1 % hydrolytic cleavage and no discoloration. Parallel photostability experiments under simulated daylight irradiation confirm that neither the linker nor its anticipated conjugates yellow or precipitate—an assurance that is critical when the final construct is destined for live-cell imaging or surface-based spectroscopy. Finally, the packaging philosophy itself must preserve the properties so painstakingly achieved: amber borosilicate vials, argon back-filling, and septa laminated with fluoropolymer barriers that resist sorption of the aromatic core. The cumulative outcome is a linker whose performance is limited only by the imagination of the chemist, not by hidden variability in the bottle.
Why Stability Is Critical in Research?
Stability is the unknown unknown that can render weeks of data worthless in an instant, after the investigator is stunned to discover that the analytical standard has isomerized unobtrusively. Aromatic linkers in particular are susceptible to three modes of decomposition: hydrolysis of pendant activated esters, photo-oxidation of electron-rich arenes, and metal-catalyzed cross-linking that transforms a monofunctional spacer into an insoluble polymer. Each mechanism is pernicious because the initial products are often spectroscopically invisible: a trace amount of quinone methide or chloroanil adduct will not change the retention time in an analytical HPLC trace, but will quench fluorescent reporters or occlude antibody binding sites. Once the construct is deployed in a biological setting the ramifications are multiplied. Serum esterases can snap an unstable linker in minutes, releasing the payload before it should, and frustrating the interpretation of pharmacokinetic data. In materials science, surface-immobilized ligands that leach off through linker fragmentation will generate false negatives in binding assays and artefactual drift in refractive-index sensors. Even for purely spectroscopic applications, photodegradation produces radical fragments that attack neighboring chromophores, shortening measurable lifetimes and skewing Förster distance calculations. Premium stability is thus an insurance policy against cascading experimental noise. It enables longitudinal measurements—week-long live-cell imaging, repeated surface regeneration cycles, or iterative chemical transformations on the same microarray spot—without the need to recalibrate or remake the construct. Just as important, it provides intellectual continuity: a PhD student who inherits a two-year-old bottle should see the same coupling efficiency and the same optical signature as the post-doc who first opened it. In that respect, stability is not just a chemical virtue; it is a guarantor of scientific memory, a safeguard that yesterday's benchmark remains tomorrow's baseline.
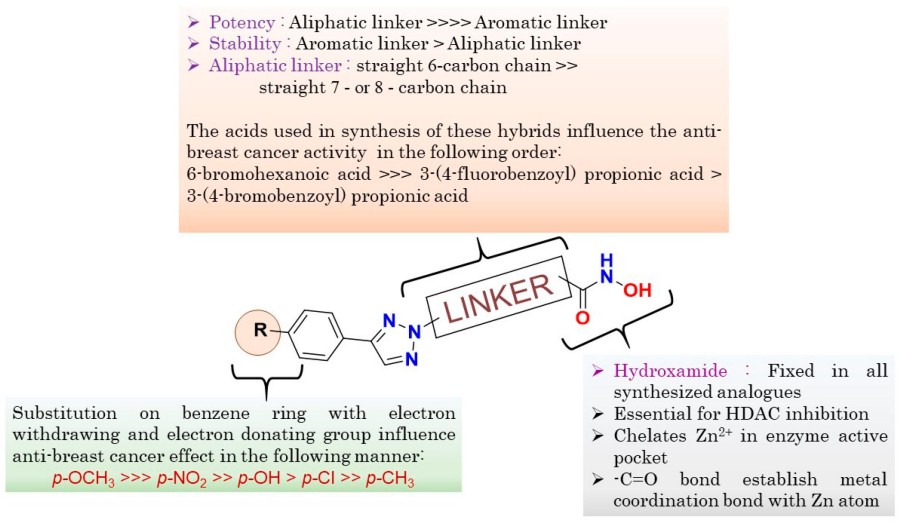 Fig. 1 General SAR of the synthesized compounds.1,2
Fig. 1 General SAR of the synthesized compounds.1,2
Product Advantages
When the synthetic route is supported by aromatic linkers whose purity pedigree is non-negotiable, the entire discovery cascade accelerates: the medicinal chemist stops fighting the reagent and starts interrogating the biology. The advantages that follow are not marketing slogans, but kinetic facts – there are fewer precipitates during conjugation, the SAR trends are crisper because off-target activity is not smeared by contaminant noise and there is a seamless translational path because the same lot that defined the nanomolar angle in March still delivers the identical ex-vivo profile in September. In short, the linker quietly removes itself from the list of experimental variables.
High Purity and Consistency Across Batches
Purity is a concept that is inherently qualitative when applied to aromatic linkers, rather than quantitative. The rationale is simple: each vial of linker must behave as if it were isolated from a single, macroscopic crystal. As such, first recrystallisation of the starting aryl halides themselves is also a purification, and the starting material is recrystallized until the 1H-NMR spectrum is "flat" apart from the aromatic proton signals expected based on the molecule's symmetry. The cross-coupling step is run under a slow-addition conditions to avoid the spectroscopically invisible, but later insoluble, grey film of homo-coupled impurity. Washes are non-polar to avoid co-extraction of palladium black during work-up, while lipophilic side-products are stripped from the desired structure, unharmed, by simple non-polar washes. Temperature-gradient sublimation of the crude material then not only removes volatiles, but separates minor isomers with slightly different melting points (differences of as little as 2˚C). This material should show a chromatographic trace that is indistinguishable from material isolated 6 months ago. This uniformity is only possible when every step of the unit operation is considered a purification, rather than isolation. Homogenisation of the final powder under an inert atmosphere prevents micro-segregation that can occur even in the static storage. The practical impact is that an investigator can validate a bioconjugation protocol on linker lot A, then later use that exact protocol on linker lot E without re-optimizing pH, stoichiometry, or incubation time (conditions, all, that may not be optimized due to narrow assay windows or precious biological material).
Reliability in SAR Studies and Scale-Up
Structure–activity relationships are stories written by small numbers: the gain or loss of one methylene should be reflected by a fold-shift in potency, not a smear of uncertainty. A linker that leaks parts-per-thousand of a regioisomeric side-chain is a second parallel SAR that muddies the waters, forcing medicinal chemists to chase ghosts. Reliability therefore starts with the absence of such phantoms; it proceeds with the guarantee that the electronic demand of the aromatic core is the same from milligram to kilogram. It is enabled by controlling the halide dance—literally the transient palladate species with room to shuffle substituents—through inverse addition and low-temperature quenches. The same protocol is hard-coded into the scale-up standard operating procedure, so that the 100 g batch is not a reckless extrapolation but a linear magnification. Stability data generated at 45 °C for eight weeks verify that no new spots come online, meaning that the linker that emerges from the GMP suite is chemically congruent with the one that first seduced the SAR team. It follows that the cellular degradation profile recorded in the 96-well plate can be reproduced in the hollow-fiber assay, and the pharmacokinetic jump from mouse to cynomolgus is not thrown off course by an unexpected impurity spike. In that sense, the linker is a perfect translator: it ferries the message from medicinal chemistry to process development without dropping a single letter.
Compatibility with Common PROTAC Scaffolds
A rod linker must rigidly present two termini with steric profiles that are matched to a warhead and an E3 ligand recruiter, without itself π-stacking to either of these domains or promoting untimely hydrolysis. PROTAC linkers must conform to this architectural grammar, and aromatic linkers fulfill it: torsional rigidity enforces a well-defined end-to-end vector, and the π-cloud is electronically shielded by fluoro or trifluoromethyl substitution, which sterically repels aromatic–aromatic stacking. The linker should also be resilient to aqueous DMSO solutions during ternary-complex formation, so the etheric oxygen atoms are spaced in such a way that no continuous chain of more than three carbons is unprotected, thus eliminating any opportunities for water to attack and cleave the backbone. Exit vector geometry is another consideration: the 180° separation enforced by the para-relationship of the two functional handles precludes steric occlusion of the lysine-rich patch required for ubiquitin transfer and holds the recruited E3 ligase at arm's length from the target protein. A linker's LogD must fall in the 1–3 range, high enough to avoid rapid renal clearance but not so high as to be prone to micelle formation, which would sequester the PROTAC away from intracellular binding partners. To this end, these guidelines are hardwired into the synthetic design so the scientist who inserts the aromatic rod between, for example, a VHL ligand and a kinase inhibitor is assured that all physical properties, from hydrodynamic radius and rotational correlation time to micro-pKa, have been presettled.
Available Linker Types
The present selection is deliberately small but carefully chosen: phenyl for minimal steric footprint, biphenyl for increased π-conjugation without conformational collapse, pyridyl for modifiable metal chelation/hydrogen-bond directionality. Each core is further capped by orthogonal exit vectors - carboxyl, amino, boronic, azide - so that the same conformationally rigid core can be appended to peptides, polymers or surfaces without having to re-optimize the whole pathway. Instead of providing an encyclopedia of scaffolds, the portfolio is centred around these three validated rings, with the belief that their electronic tunability and crystallographic predictability is sufficient to span most of the design space of modern conjugation chemistry.
Phenyl, Biphenyl, and Pyridyl Linkers
Phenyl is the least opinionated of all aromatic units: its six-fold symmetry means 1,4-, 1,3- or 1,2-difunctionalisation does not break dipole symmetry and such indifference can be a virtue when the linker is not to perturb the electronics of adjacent pharmacophores. The core's planarity also confers a known 2.5 Å repeat distance, turning the phenylene into a molecular ruler for applications that require sub-ångström length control. Biphenyl relieves the torsional stiffness of phenylene by introducing a rotatable inter-aryl bond; in practice, crystallographic surveys have shown that, once the peripheral substituents clear a low steric hurdle, the dihedral angle is effectively locked to ~30–40°, which furnishes an extended π-system while still avoiding the conformational entropy of an alkyl tether. This semi-rigidity has a direct effect on molar absorptivity and yields a small but systematic increase in Förster radius, a property harnessed in long-range energy-transfer probes. Pyridyl is a departure from purely hydrocarbon chemistry: a sp² nitrogen is a perturbation that, in terms of bond lengths, is relatively small but leads to a substantial change in reactivity. The lone pair constitutes a soft metal-binding site and reversible chelation to Zn(II) or Ru(II) centers can be achieved without any separate ligands, while at the same time, the electron-poor ring lowers the pKa of neighboring amines by approximately one pKa unit, a shift that can be exploited to pH-switch release. Critically, all three cores can be interconverted in late-stage (either via Suzuki or Liebeskind cross-couplings) so that lead optimization does not need to be repeated ab initio in the event that the original spacer is too short or lipophilic.
High-Purity Phenyl Linkers - Ready for Your Next PROTAC Project
View the full catalog and specifications to explore our selection of high-purity Phenyl linkers optimized for targeted protein degradation.
Functionalized Aromatic Variants
In addition to the parent hydrocarbons, the challenge is to install handles that are reactive towards the orthogonal bioconjugation strategy, but otherwise "innocent" with respect to the downstream biology. A perennial favorite is the para-carboxy derivative, whose benzoic acid sidechain can be converted to the N-hydroxysuccinimide ester quantitatively under rigorously anhydrous conditions; the resulting activated species is stable in refrigerated storage for months, yet couples to lysine ε-amines within minutes at physiological pH. Terminal azides for copper-free click chemistry are installed via Sandmeyer-like diazotization followed by quenching with trimethylsilyl azide, a sequence that avoids shock-sensitive sodium azide used so often in the early literature. In case of redox sensitivity (e.g., for intracellular glutathione concentrations), electron-withdrawing fluorine substituents are added in the meta position; the C–F bond is resistant to reductive cleavage yet inductively enhances nucleophilic aromatic substitution should a traceless cleavage be desired in the future. Orthogonality is further broadened by adding pinacol boronates which couple under aqueous Suzuki–Miyaura conditions and thereby permit late-stage diversification on the benchtop without stringently dried glassware. Finally, for photochemical applications, o-nitrobenzyl esters are added to the same cores; these caging groups absorb at 365 nm, a wavelength that is innocuous to most proteins, and fragment with quantum efficiencies high enough to permit micromolar uncaging doses. The overlying design philosophy is to view the aromatic nucleus as an electronic breadboard: the π-system is held constant, while peripheral substituents are interchanged to address the idiosyncrasies of each new conjugation campaign.
Logistics and Support
At BOC Sciences, we combine scientific precision with dependable logistics to ensure that every aromatic linker reaches your lab quickly, safely, and in perfect condition. Our global infrastructure and responsive support team help research organizations streamline procurement and focus on what truly matters -advancing discovery.
Global Inventory and Fast Shipping
We maintain a worldwide inventory of high-purity aromatic linkers, ready for immediate dispatch to academic, biotech, and pharmaceutical partners.
- Regional warehouses in key markets allow for same-day or next-day shipping.
- Temperature-controlled logistics protect sensitive compounds during transit.
- All shipments include tracking information and are packaged according to IATA and UN transport standards for chemical safety.
Our distribution model minimizes lead time and ensures reliable access to essential PROTAC-building components across North America, Europe, and Asia.
Documentation: COA, NMR, LC-MS Data
Every product we ship comes with complete quality documentation to guarantee traceability and reproducibility.
Each batch includes:
- A detailed Certificate of Analysis (COA) verifying identity and high purity.
- Analytical spectra: HPLC chromatograms, LC-MS data, and 1H/13C NMR spectra.
- Batch traceability and storage stability information for compliance reporting.
These records empower researchers to maintain consistent analytical standards and facilitate straightforward regulatory submission or publication support.
Ordering Options
We make it easy to source premium aromatic linkers-whether you need a small sample for exploratory chemistry or bulk material for preclinical programs.
Ready-to-Use SKUs
Our catalog features an extensive range of ready-to-use aromatic linkers, including:
- Phenyl, biphenyl, pyridyl, and heteroaryl scaffolds.
- Multiple reactive handles (bromo, iodo, azide, alkyne, boronic acid).
- Standard packaging sizes from 100 mg to 10 g for fast, small-scale R&D.
Custom Synthesis and Bulk Discounts
For teams needing tailor-made aromatic linkers, we offer custom synthesis to adjust substitution patterns, electronic properties, or conjugation functionality.
- Design and synthesis of novel aromatic frameworks on demand.
- Bulk discounts and multi-batch production scheduling for high-volume customers.
- Expert technical consultation to help identify the best linker structure for your specific target or E3 ligase system.
Our dedicated chemists ensure every custom order meets your scientific and operational expectations - from purity verification to delivery timelines.
Partner with Us for Premium Aromatic Linkers
High-purity aromatic linkers are vital for creating rigid, stable, and efficient PROTAC molecules. Choosing a trusted supplier ensures that every compound you receive is analytically verified, performance-proven, and ready to support high-impact research. At BOC Sciences, we take pride in delivering premium aromatic linkers that combine chemical excellence, batch consistency, and global availability. From stocked phenyl and pyridyl linkers to fully custom synthesis projects, we deliver reliability at every stage.
Contact our sales or technical support team today to request a quote, explore bulk discounts, or start a custom aromatic linker project tailored to your research goals. Empower your discovery pipeline with chemistry you can trust - built for innovation, quality, and speed.
FAQs
1. Can I order bulk or custom aromatic linkers?
Yes, we provide both ready-to-use SKUs and custom synthesis options with volume discounts.
References
- Image retrieved from Figure 1 " General SAR of the synthesized compounds," Shirbhate E.; et al., used under [CC BY 4.0](https://creativecommons.org/licenses/by/4.0/). The original image was not modified.
- Shirbhate E, Koch B, Singh V, et al. Heteroaryl-Capped Hydroxamic Acid Derivatives with Varied Linkers: Synthesis and Anticancer Evaluation with Various Apoptosis Analyses in Breast Cancer Cells, Including Docking, Simulation, DFT, and ADMET Studies[J]. Pharmaceuticals, 2025, 18(8): 1148. https://doi.org/10.3390/ph18081148.
- Zagidullin A, Milyukov V, Rizvanov A, et al. Novel approaches for the rational design of PROTAC linkers[J]. Exploration of Targeted Anti-tumor Therapy, 2020, 1(5): 381. https://doi.org/10.37349/etat.2020.00023.
- Bricelj A, Steinebach C, Kuchta R, et al. E3 ligase ligands in successful PROTACs: an overview of syntheses and linker attachment points[J]. Frontiers in chemistry, 2021, 9: 707317. https://doi.org/10.3389/fchem.2021.707317.

 Fig. 1 General SAR of the synthesized compounds.1,2
Fig. 1 General SAR of the synthesized compounds.1,2