Over the past two decades, a growing number of scientists have begun to develop novel drug delivery systems for a variety of drugs with poor physical and chemical properties to improve their solubility, intracellular accumulation, and site-specific distribution while minimizing side effects. The drug delivery system helps PROTAC avoid hydrophobicity, increase bioavailability, and significantly enhance drug properties thanks to its loading capacity, longer cycle times, and targeting capabilities.
1. Protein-based delivery systems
Protein-based delivery systems can transport drugs to specific locations because of their tertiary structure-mediated receptor-ligand recognition. Therefore, protein-based delivery designs can be applied to PROTAC.
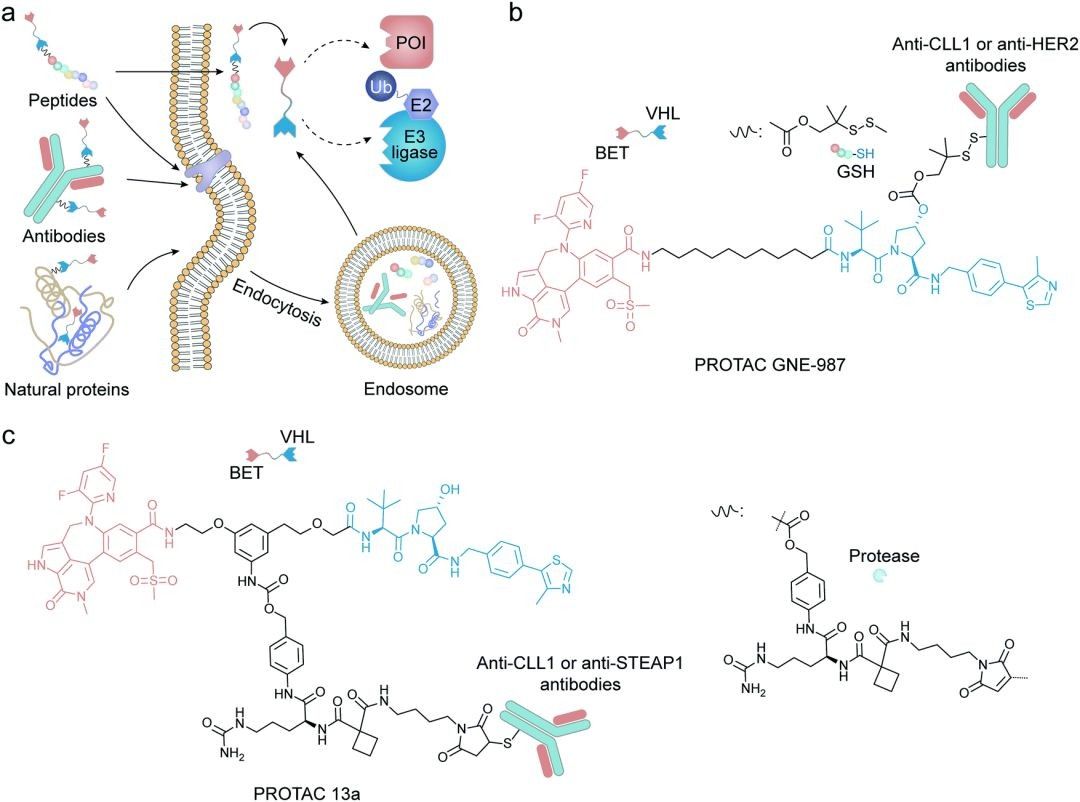 Fig 1. Protein degradation induced by a protein-based PROTAC delivery system1
Fig 1. Protein degradation induced by a protein-based PROTAC delivery system1
1.1 Antibody-based delivery systems
Antibodies are used by the adaptive immune system to recognize and neutralize pathogens. They enjoy high affinity and specificity for their targets and have previously been used as targeting ligands for drug delivery vectors. Antibody-drug conjugates (ADCs) are more common than other protein-based drug delivery methods. ADCs combine the targeting and long-cycle properties of antibodies with therapeutic agents to minimize the accumulation of drugs in normal tissues to reduce off-target side effects. Inspired by ADC, Pillow et al developed the first antibody-PROTAC conjugate, linking BET depressant (GNE-987) to anti-ClL1 or anti-HER2 antibodies to overcome their adverse physicochemical properties and pharmacokinetic properties. Using a disulfide-containing linker, GEN-987 was derived by forming a carbonate bond on the hydroxyl group of the proline site in the VHL ligand. The half-life of PROTAC was significantly prolonged by intravenous administration after the derivatives were linked to cystine of the anti-ClL1 antibody.
1.2 Peptide-based delivery systems
Although antibody-PROTAC conjugates have many advantages, most molecules are immunogenic and difficult to cross physiological barriers. In contrast, peptides with low molecular weight and less immunogenicity can serve as ideal substitutes for these antibody-PROTAC conjugates because they can perform similar functions to their larger protein counterparts. Researchers have begun to design and construct a peptide-based vector for PROTAC delivery. Rodriguez-Gonzalez used polyarginine cell-penetrating peptide (CPP) to modify PROTAC targeting AR and ER for the treatment of breast and prostate cancer, respectively.
In addition to the antibody- and peptide-based drug delivery systems described above, many natural proteins such as albumin, ferritin, and transferrin also have the potential to transport PROTAC molecules. Individual protein molecules have hydrophobic cavities that can encapsulate or bind PROTAC. Many proteins can package PROTACs artificially or by self-aggregating to form aggregates. In addition, monomers and aggregates can also be chemically or biologically modified to further enhance their functional or pharmacokinetic properties. This also facilitates PROTAC loading, delivery, release, and synergistic efficacy. However, the problems raised by protein- or peptide-based PROTAC delivery systems cannot be ignored. Examples include protein- or peptide-induced toxicity, such as hematotoxicity and immunogenicity. In addition, degradation of the protein carrier during the cycle may also lead to premature release of PROTAC, resulting in reduced therapeutic efficacy and off-target side effects.
2. Nucleic acid-based delivery systems
Unmodified nucleic acids usually require delivery systems for their therapeutic effects but, recent advances in technology targeting deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) have inspired a new direction in nucleic acids as delivery systems by revealing new functions and physicochemical/biochemical properties of nucleic acids. For example, aptamers and spliced single-stranded DNA or RNA molecules are selected to bind to specific targets using the complementary pairing principle of base pairs. Similar to the folding of peptides into proteins, nucleic acids fold into different secondary structures, which are then further modified to form unique three-dimensional structures that recognize their targets. Aptamers can direct drugs to the desired cell or tissue due to their high affinity and specificity.
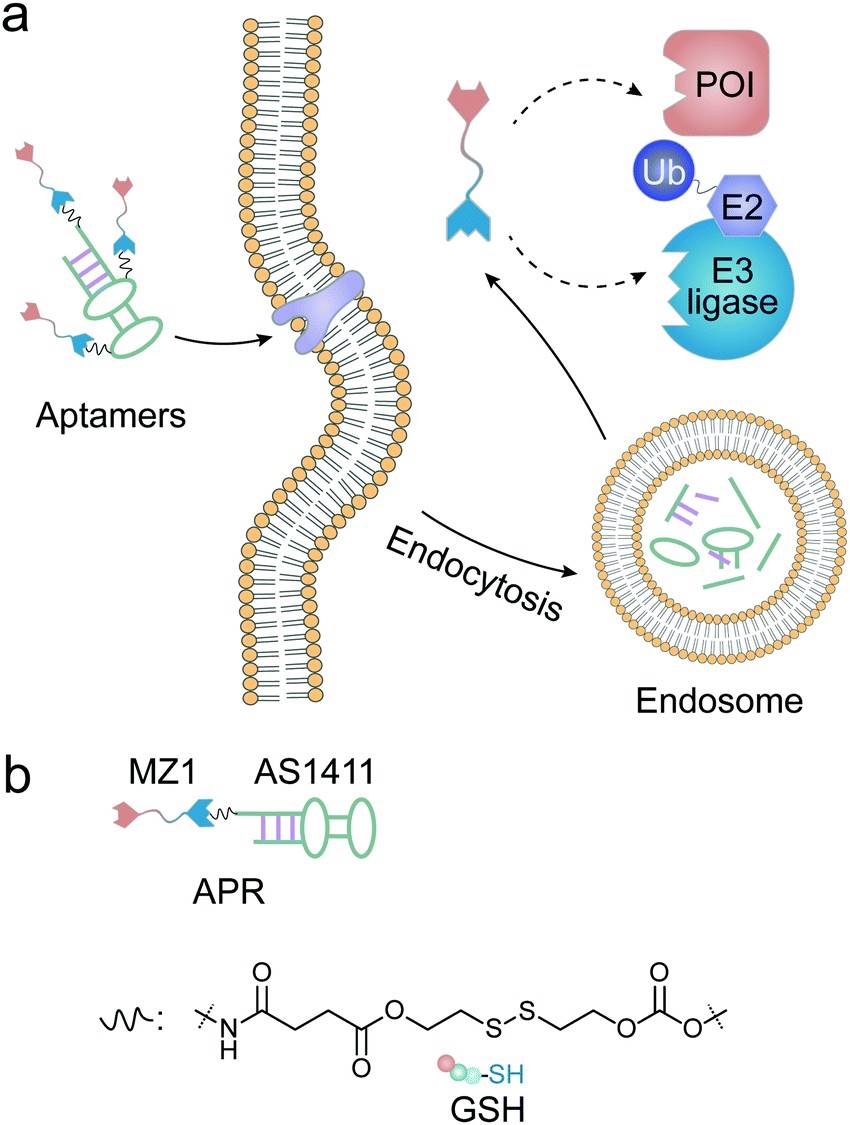 Fig 2. Protein degradation mediated by aptamer-PROTAC conjugate1
Fig 2. Protein degradation mediated by aptamer-PROTAC conjugate1
The development of the PROTAC delivery systems based on nucleic acid is still in its infancy and it faces some problems. The half-life of the PROTAC delivery system of nucleic acid is greatly reduced due to its instability in the systemic circulation, which can be cleared by rapid metabolism. In addition, the immune system can recognize these nucleic acids and may induce an excessive immune response. Nucleic acid delivery vectors may also be affected by the polyanion effect leading to potential toxic side effects.
3. Liposome-based nanoparticles
Liposome is a multifunctional vector composed of a large number of substructures. Most lipid-based nanoparticles are spherical vesicles, which often contain uniformly multiple water-soluble components within the lipid bilayer. Liposomes as drug delivery systems have many advantages, such as simple formulation, good biocompatibility, high bioavailability, excellent loading capacity, and flexible and adjustable physicochemical properties. A Chinese research group developed a liposome capable of simultaneously carrying a protein kinase C inhibitor, palmitoyl-DL-carnitine chloride (PC), and a BRD4-targeting PROTAC - ARV. It's named LARPC.
Because of the lipophilic palmitoyl chain and hydrophilic carnitine head group, the palmitoyl chain and hydrophobic ARV of PC were located in the lipid bilayer. LARPC had a particle size of 105.25 ± 2.76 nm, and the liposome can selectively aggregate in tumor sites due to its high permeability and EPR effect. The positive surface charge of 26.6 mV promoted its penetration into tumor tissues and enhanced its ability to enter cancer cells. LARPC had shown effective inhibition of melanoma cell lines, which provided a prospect for the use of liposome PROTAC in melanoma treatment. Liposomes are usually composed of phospholipids that form monolayer and multilayer vesicle structures. These nanoparticles are composed of the lipid bilayer and water chamber, enabling them to encapsulate both hydrophilic and hydrophobic drug molecules. Due to poor water solubility, most PROTAC molecules can only be loaded into phospholipid bilayer rather than large water-based chambers, which calls into question the potential of liposomes for PROTAC applications.
However, PROTACs can be chemically modified with hydrophilic groups to alter their solubility, which can increase the loading of PROTACs in the hydrophilic core of lipid nanoparticles. The approval of the liposome vaccine COVID-19 has increased the awareness that the integration of PROTAC with lipid nanoparticles can promote the clinical transformation of PROTAC.
Summary
The development of PROTAC delivery systems can accelerate its transformation from experimental to clinical. These delivery systems can optimize the physicochemical properties of PROTAC, achieve targeted delivery, promote intracellular accumulation, and enhance its degradation potency. Thanks to the delivery system's superior load capacity, PROTAC can also be co-delivered with other therapies to achieve the goal of combination therapy. In addition, by introducing a stimulus-response mechanism, PROTACs can be continuously released from the delivery system under specific temporal and spatial conditions, which can solve the unpredictability of dose caused by the Hook effect. By combining advanced technologies from multiple interdisciplinary fields, such as materials science, chemistry, and bioengineering, PROTACs' delivery systems can be further optimized and expanded, and customized into precision medicine to meet the treatment needs of each disease.
Reference
- Chen, Y., et al. Proteolysis-targeting chimera (PROTAC) delivery system: advancing protein degraders towards clinical translation, Chem. Soc. Rev., 2022,51, 5330-5350.

 Fig 1. Protein degradation induced by a protein-based PROTAC delivery system1
Fig 1. Protein degradation induced by a protein-based PROTAC delivery system1 Fig 2. Protein degradation mediated by aptamer-PROTAC conjugate1
Fig 2. Protein degradation mediated by aptamer-PROTAC conjugate1