The immunomodulatory imide drug (IMiD) family has become a cornerstone in modern targeted protein degradation, offering unparalleled utility in both molecular glue discovery and PROTAC design. As high-value cereblon (CRBN) ligands, IMiD compounds—most notably thalidomide, lenalidomide, and pomalidomide—enable precise E3 ligase recruitment and programmable protein elimination across a wide range of biological systems. Their well-defined structural motifs, predictable CRBN interactions, and exceptional linker-attachment flexibility have positioned IMiDs as the industry's most reliable starting point for designing next-generation degraders. Today, with expanding demand for safer analogs, improved selectivity, and computationally optimized chemotypes, IMiDs continue to reshape the landscape of drug discovery and chemical biology. This article provides a comprehensive overview of their mechanisms, functional benefits, emerging trends, and the IMiD-based products and services we offer to support your PROTAC innovation pipeline.
Overview of the IMiD Family
Immunomodulatory imide drugs (IMiDs) represent a cornerstone class of small molecules widely used in targeted protein degradation and E3 ligase-based therapeutic design. As cereblon (CRBN) ligands, IMiD compounds have become indispensable tools for understanding ubiquitin-proteasome biology and for engineering heterobifunctional degraders such as PROTACs. Their well-characterized structure-activity relationships, predictable binding behavior, and broad commercial availability make IMiDs the most mature and reliable CRBN-recruiting chemotypes in modern drug discovery.
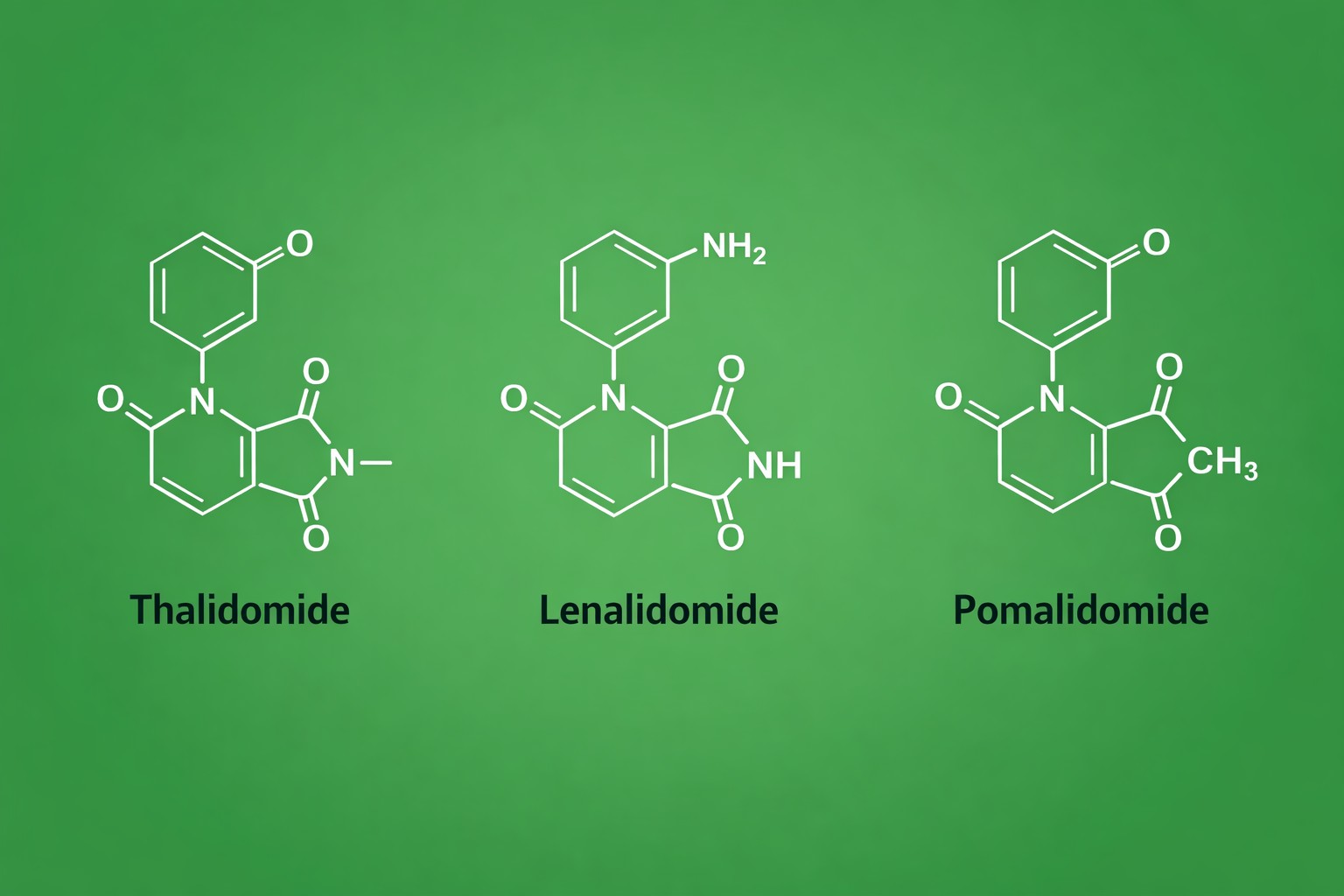 Fig 1. Chemical structure comparison of thalidomide, lenalidomide, and pomalidomide
Fig 1. Chemical structure comparison of thalidomide, lenalidomide, and pomalidomide
Thalidomide, Lenalidomide, and Pomalidomide at a Glance
The IMiD family is historically anchored by three clinically validated molecules—thalidomide, lenalidomide, and pomalidomide. Each compound shares a common phthalimide-glutarimide core, yet subtle chemical modifications confer distinct biological properties:
- Thalidomide: The first-generation IMiD and the parent scaffold for CRBN ligands. Although notorious for its teratogenicity, it provides the structural blueprint for cereblon engagement.
- Lenalidomide: Designed to enhance immunomodulatory effects and reduce adverse outcomes. Its improved potency in CRBN binding and neosubstrate recruitment makes it highly effective in hematological malignancies.
- Pomalidomide: A third-generation analog with further optimized activity and pharmacokinetics. Pomalidomide exhibits strong CRBN affinity and robust downstream signaling effects, making it a preferred starting point for high-efficiency PROTAC design.
Collectively, these thalidomide analogs offer an evolution of potency, selectivity, and chemical versatility—attributes that directly translate into their success as cereblon-recruiting ligands.
Table 1: Comparison of Core IMiD Compounds: Thalidomide, Lenalidomide, and Pomalidomide
| Compound | Generation | Key Structural Feature | CRBN Binding Strength | Exit Vector Options | Neosubstrate Engagement | Typical Applications |
| Thalidomide | 1st | Parent IMiD scaffold | Moderate | Limited | High (e.g., SALL4 risk) | Basic CRBN studies, glue mechanism |
| Lenalidomide | 2nd | Amino-substituted ring | High | Good | Strong IKZF1/3 degradation | Oncology models, CRBN assay systems |
| Pomalidomide | 3rd | Optimized phthalimide | Very High | Excellent | Robust IKZF recruitment | High-potency PROTAC design |
Shared Pharmacophore and Structural Motifs
Despite their functional differences, IMiDs maintain a conserved pharmacophore essential for CRBN recognition. The key structural motifs include:
- The glutarimide ring, which serves as the primary CRBN-binding element, fitting deeply into the hydrophobic tri-tryptophan pocket of cereblon.
- The phthalimide (or substituted isoindolinone) ring, which extends toward solvent and can be selectively functionalized without disrupting CRBN affinity.
- Defined stereochemistry at the glutarimide, critical for stable and high-affinity binding.
These unified structural features enable predictable interactions with CRBN while leaving defined positions available for linker attachment—one of the major reasons IMiD compounds have become the industry standard for E3 ligase recruitment. Their modularity supports rapid analog generation, structure-guided optimization, and consistent performance across diverse PROTAC architectures.
Mechanisms of CRBN Binding and Activation
A defining feature of IMiD compounds is their ability to bind cereblon (CRBN), the substrate receptor of the CUL4-RING E3 ligase complex. This interaction not only stabilizes CRBN in its active conformation but also enables the recruitment of neosubstrates or engineered target proteins in PROTAC systems. Understanding the molecular basis of IMiD-CRBN recognition is essential for rational degrader design and for developing next-generation thalidomide analogs with improved specificity and safety.
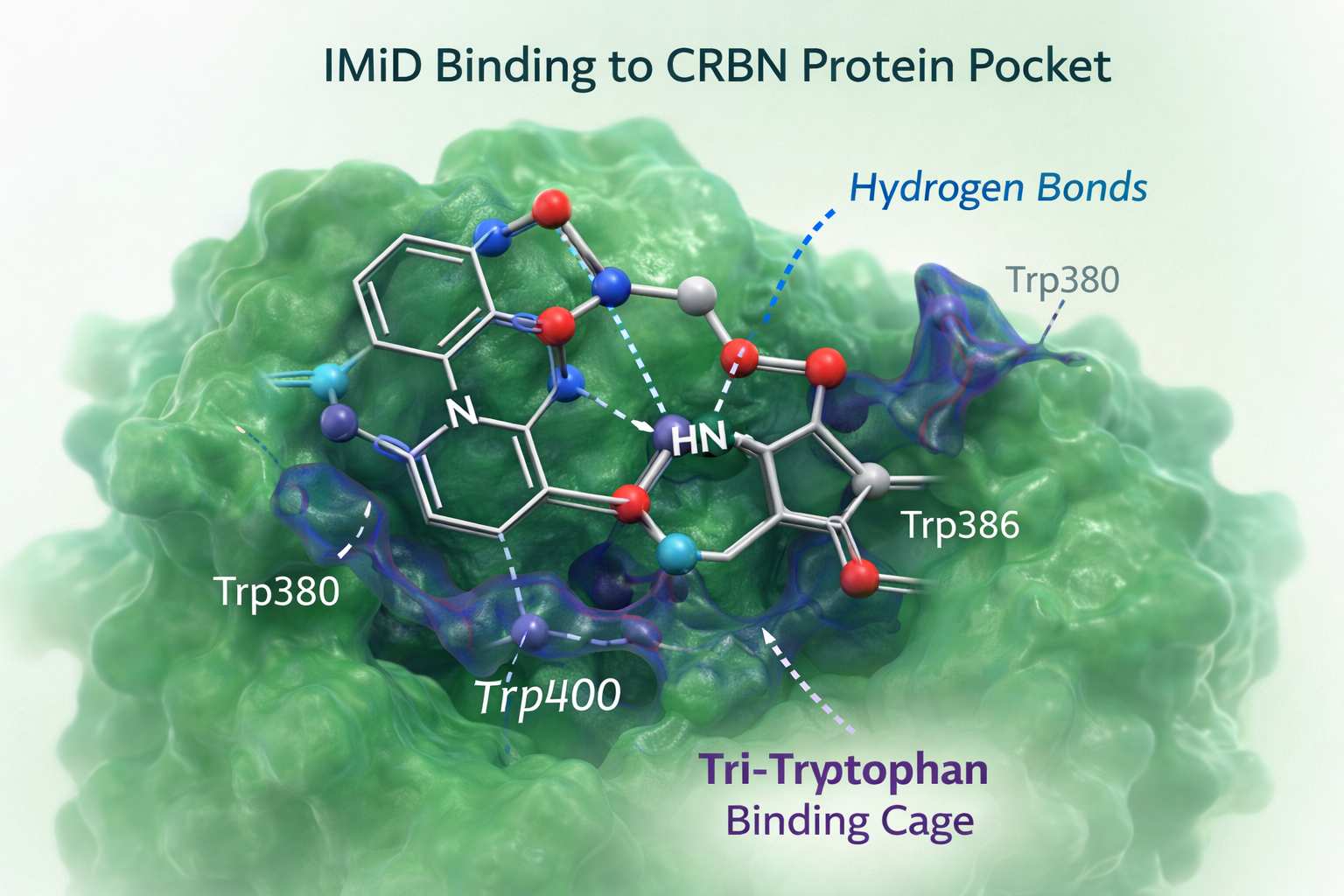 Fig 2. IMiD binding to the CRBN protein pocket
Fig 2. IMiD binding to the CRBN protein pocket
Hydrogen Bonding and Binding Pocket Interactions
IMiDs bind CRBN through a set of highly conserved interactions within the protein's tri-tryptophan (Trp) binding pocket. Several structural features drive this high-affinity engagement:
- Glutarimide-CRBN hydrogen bonding: The glutarimide ring forms critical hydrogen bonds with CRBN residues, anchoring the IMiD deep within the binding pocket. These interactions serve as the primary determinant of cereblon ligand affinity.
- Hydrophobic contacts with aromatic residues: Three key tryptophan residues create a hydrophobic cage that stabilizes the ligand. This unique environment shapes the selectivity of CRBN for IMiD compounds compared with other E3 ligase systems.
- Orientation of the phthalimide ring: The phthalimide (or isoindolinone) moiety protrudes toward the solvent-exposed region, enabling additional van der Waals interactions and providing chemical space for linker attachment in PROTACs.
Together, these interactions ensure that even small structural variations in IMiDs can significantly influence CRBN binding strength and downstream degradation activity.
Why IMiDs Exhibit High Ligase Affinity
The high ligase affinity observed across IMiD compounds results from a balance of structural complementarity and conformational stability:
- Evolutionary optimization: Thalidomide analogs have been refined over decades, indirectly selecting for enhanced CRBN engagement in clinical and research contexts.
- Rigid core scaffold: The relatively rigid IMiD framework minimizes entropic penalties during binding, supporting strong and predictable ligand-receptor interactions.
- Favorable physicochemical profile: Their moderate polarity and size allow IMiDs to diffuse effectively into intracellular compartments where CRBN resides, ensuring efficient ligase recruitment in live-cell assays and therapeutic applications.
- Consistent SAR trends: Predictable structure-activity relationships simplify rational modification without compromising CRBN affinity—a key advantage in PROTAC design workflows.
These properties collectively explain why IMiDs remain the industry's most reliable cereblon ligands and why they continue to underpin the majority of CRBN-targeted degrader platforms.
Functional Benefits in PROTAC Design
In the rapidly evolving field of targeted protein degradation, IMiD-based cereblon ligands remain the gold standard for PROTAC development. Their predictable chemical behavior, excellent ligandability, and robust E3 ligase recruitment capacity allow medicinal chemists to design degraders with high efficiency and minimal synthetic complexity. These functional benefits make IMiD compounds indispensable tools in translational research, drug discovery pipelines, and preclinical validation studies.
Ease of Derivatization and Linker Attachment
One of the most practical advantages of IMiD compounds is the availability of well-defined positions for chemical modification without compromising CRBN affinity. This synthetic flexibility comes from the phthalimide/isoindolinone moiety, which tolerates substitution at several solvent-exposed sites.
Key benefits include:
- Established exit vectors: Specific positions—most commonly the C4 or C5 site—can be functionalized to introduce alkyl chains, PEG linkers, or click-ready handles, enabling rapid assembly of diverse PROTAC libraries.
- Minimal disruption to binding: Because the glutarimide ring anchors the ligand to CRBN, modifications on the opposite aromatic ring rarely interfere with binding kinetics or biological activity.
- Scalability and reproducibility: IMiD analog derivatization is highly reproducible across research groups, facilitating consistent production for screening and optimization campaigns.
This synthetic tractability significantly reduces development timelines, allowing teams to iterate rapidly on linker length, orientation, and degradation profiles.
Compatibility with Diverse Target Ligands
IMiD-based cereblon ligands pair effectively with a broad spectrum of target-binding warheads, making them exceptionally versatile in PROTAC design. Their predictable geometry and compact structure support favorable ternary complex formation, even with large or structurally complex target molecules. Functional advantages include:
- High success rate in forming productive ternary complexes: IMiDs often promote cooperative interactions between CRBN and the target protein, enhancing degradation efficiency even for difficult-to-drug targets.
- Compatibility with multiple ligand classes: They can be coupled to reversible inhibitors, covalent binders, peptidomimetics, or fragment-based ligands, providing broad applicability across therapeutic areas.
- Robust performance in cellular assays: IMiD-based PROTACs frequently exhibit strong cell permeability and sustained intracellular CRBN engagement, supporting potent and selective target degradation.
For discovery teams aiming to validate targets quickly or accelerate hit-to-lead progress, IMiDs offer a reliable and adaptable platform for ligase recruitment.
Dual Nature—Molecular Glues and Ligase Recruits
IMiD compounds occupy a unique position in drug discovery because they function both as classical cereblon ligands for PROTAC construction and as molecular glues that induce neosubstrate degradation on their own. This dual nature has reshaped how scientists conceptualize E3 ligase recruitment—expanding the field beyond bifunctional degraders and into small-molecule-driven rewiring of protein-protein interactions. Understanding these two roles is essential for designing safe, effective, and selective IMiD-based therapeutics and research tools.
Understanding Neosubstrate Degradation
As molecular glues, IMiDs promote the recruitment and degradation of specific endogenous proteins, referred to as neosubstrates. This process occurs independently of a PROTAC's bifunctional architecture and relies entirely on the IMiD-CRBN interface altering CRBN's substrate specificity. Key aspects include:
- Recruitment of transcription factors: IMiDs facilitate CRBN-mediated degradation of IKZF1/3 (Ikaros and Aiolos), central to immunomodulatory and anticancer activity.
- Allosteric modulation of CRBN: Binding of an IMiD reshapes the ligand-binding pocket, creating novel recognition surfaces for neosubstrates.
- Therapeutic implications: The biological effects of lenalidomide and pomalidomide in hematologic malignancies stem directly from targeted IKZF degradation.
For PROTAC developers, awareness of these inherent neosubstrate activities is crucial for anticipating potential off-target effects, understanding cellular context, and designing more selective ligase-recruiting moieties.
Table 2: Comparison of IMiD Function as Molecular Glues vs. PROTAC CRBN Ligase Recruits
| Feature | Molecular Glue Behavior (IMiDs Alone) | PROTAC Ligase Recruit Function |
| Mode of Action | Induce neosubstrate degradation | Bring target protein to CRBN via bifunctional linker |
| Requirement for Targeting Ligand | None | Requires warhead + linker |
| Typical Substrates | IKZF1/3, CK1α, etc. | Designed for any chosen target |
| Advantages | Simple molecule, high potency | High selectivity, programmable degradation |
| Risks / Considerations | Unintended neosubstrate effects | Ternary complex dependency |
Balancing Efficacy and Selectivity in Design
Because IMiDs can act both as PROTAC recruiters and as molecular glues, achieving the right balance between efficacy and selectivity is a central design challenge. Several considerations guide optimal engineering:
- Managing neosubstrate liabilities: Designer IMiDs or non-degrading CRBN ligands may be preferred when avoiding IKZF1/3 engagement is critical for safety or specificity.
- Fine-tuning linker geometry: Proper orientation and length of the linker can modulate ternary complex formation, enhancing target selectivity while minimizing unintended degradation events.
- Leveraging structure-guided design: Computational modeling and cryo-EM structures help predict how modifications influence the ternary complex, allowing chemists to optimize potency without triggering undesired molecular glue activity.
- Exploring next-generation CRBN ligands: Novel analogs with reduced neosubstrate recruitment profiles are emerging to provide safer and more selective alternatives for PROTAC applications.
Ultimately, the dual nature of IMiDs offers both opportunities and challenges. Harnessing their molecular glue behavior can unlock new biology, while careful design ensures that ligase recruitment occurs with precision and control.
Emerging Trends and Future Outlook
As the field of targeted protein degradation continues to evolve, IMiD-based cereblon ligands remain indispensable tools—but they are far from static. Researchers are now developing next-generation analogs with improved safety, broader chemical diversity, and enhanced compatibility with advanced computational design pipelines. These innovations aim to overcome the limitations of classical thalidomide analogs while opening new doors for precise, programmable protein degradation.
New Non-Teratogenic IMiD Analogs
Recent advances in IMiD chemistry have focused on the development of non-teratogenic cereblon ligands that preserve high CRBN affinity while reducing safety liabilities associated with classical thalidomide analogs. By modifying key elements of the glutarimide or phthalimide core, or by introducing alternative scaffolds that mimic the canonical IMiD binding geometry, researchers aim to minimize unwanted neosubstrate recruitment such as SALL4 degradation, which is closely linked to teratogenic risk. These next-generation IMiD analogs are designed to offer improved selectivity, reduced off-target effects, and enhanced suitability for both research and therapeutic PROTAC applications. As a result, they provide safer and more versatile options for E3 ligase recruitment, particularly in early-stage discovery programs where precise control over degradation profiles is critical.
Computational Approaches in Ligand Engineering
In parallel with chemical innovation, computational approaches have become integral to the rational engineering of IMiD-based cereblon ligands. Structure-based modeling, molecular docking, and molecular dynamics simulations are increasingly used to predict CRBN binding modes, optimize exit vector positioning, and evaluate ternary complex stability prior to synthesis. Machine learning and AI-driven platforms further accelerate ligand optimization by identifying favorable structure-activity relationships and prioritizing modifications that enhance degradation efficiency while limiting undesired neosubstrate effects. Combined with high-resolution structural data from X-ray crystallography and cryo-EM studies, these computational tools enable more precise, efficient, and data-driven design of next-generation IMiD ligands, significantly shortening development cycles and improving the success rate of PROTAC discovery.
Our IMiD-Based Products and Services
As a trusted partner in targeted protein degradation research, we offer a comprehensive suite of IMiD-based products and scientific services designed to accelerate PROTAC discovery and optimization. Our portfolio spans high-purity CRBN ligands, custom synthetic solutions, and full analytical support—ensuring researchers can advance from concept to validated degrader with confidence and efficiency.
Full Range of IMiD Derivatives for Research Use
We supply an extensive catalog of IMiD-based cereblon ligands and functionalized building blocks tailored to the needs of medicinal chemists and degrader biologists:
- Thalidomide, lenalidomide, pomalidomide scaffolds: Offered in research-grade purity and multiple functional variants for direct PROTAC synthesis.
- Pre-functionalized IMiD intermediates: Available with alkyne, azide, amine, PEG, and other widely used handles suitable for rapid conjugation.
- Non-teratogenic and next-generation CRBN ligands: Engineered to support selective studies, reduced neosubstrate degradation, or enhanced linkability.
- Bulk synthesis and lot consistency: Ideal for high-throughput screening, optimization campaigns, or scale-up research.
By maintaining rigorous quality control standards, we ensure every batch meets strict specifications for purity, identity, and performance.
Custom Functionalization and Analytical Support
For advanced degrader programs requiring unique molecular architectures, our team provides tailored solutions:
- Custom IMiD derivatization: We design and synthesize bespoke cereblon ligands with specialized exit vectors, linkers, or physicochemical profiles.
- PROTAC-ready building block development: Whether you need novel attachment points, orthogonal protective groups, or unique structural motifs, we deliver optimized intermediates for reliable ligase recruitment.
- Analytical characterization: Comprehensive NMR, LC-MS, HPLC purity data, and stability testing ensure full traceability and confidence in your materials.
- Structure-activity consultation: Our scientists offer expert guidance on CRBN affinity, linker placement, and PROTAC architecture to support rational design and rapid troubleshooting.
With an integrated chemistry and analytical platform, we help researchers de-risk early-stage development and improve downstream performance.
Advantages: Proven Expertise, Reliable Supply, Technical Collaboration
Partnering with us provides more than high-quality IMiD derivatives—it offers a strategic extension of your internal capabilities. Our key strengths include:
- Industry-experienced scientific team: Specialists in PROTAC design, E3 ligase biology, and medicinal chemistry who understand real-world development challenges.
- Reliable global supply chain: We maintain stable inventory, consistent production, and dependable lead times to keep your research running uninterrupted.
- Collaborative problem-solving: From compound selection to degradation assay troubleshooting, our experts work alongside your team to accelerate discovery and optimization.
- Regulatory and documentation support: Certificates of analysis, safety documents, and data packages are prepared to meet the needs of academic labs, biotech startups, and pharmaceutical R&D groups.
By combining technical depth with operational excellence, we enable researchers to pursue innovative protein degradation strategies with confidence and speed.
Advance Your PROTAC Research with High-Quality IMiD and Cereblon Ligands
Whether you are initiating a new PROTAC program, optimizing an existing degrader series, or evaluating next-generation cereblon ligands, our IMiD-based products and tailored scientific support can significantly accelerate your discovery timeline. We invite you to explore how our expertise, reliability, and collaborative approach can empower your research.

 Fig 1. Chemical structure comparison of thalidomide, lenalidomide, and pomalidomide
Fig 1. Chemical structure comparison of thalidomide, lenalidomide, and pomalidomide Fig 2. IMiD binding to the CRBN protein pocket
Fig 2. IMiD binding to the CRBN protein pocket