Monodisperse PEG spacers have become the unassuming foundation of modern degradation campaigns. However, this leverage is lost if sub-stoichiometric amounts of trace impurities are carried forward to biological assays. A small fraction of either shortened or over-oxidized oligomer can out-compete the native degrader for either the protein or the box, seed soluble aggregates that artifactually increase cytotoxicity, or activate innate-immune sensing of ethylene glycol oligomers. High-purity lots therefore serve less as a luxury specification than an experimental prerequisite, one that compresses the variance of SAR tables, speeds regulatory analytical validation, and preserves the credibility of structure–activity interpretations that will guide second-generation rigidification or stereochemical editing. In short, the linker is no longer a passive tether; it is an allosteric modulator whose precise length, end-group integrity and batch-to-batch consistency determine whether a library provides meaningful degradation vectors or merely noisy IC50 curves.
Why Purity Matters in PROTAC Linkers?
There are three interfaces that define the activity of a bifunctional degrader, the POI warhead, the E3 ligand, and the spacer that sits between the two. Of these three, only the spacer is designed synthetically as opposed to evolved. Protein context will naturally accommodate variation at the other two points of interface, but the link is another story. Oversized impurities effectively reduce the molarity of the ternary complex, and shorter truncation products lock into non-productive binary interactions, in both cases lowering the apparent DC50 and potentially skewing evaluation at the lead optimization stage. Worse still, any polydispersity in a PEG batch adds a degree of variation to its hydrodynamic radius, throwing off the power law relationships that govern PK in plasma and thus introducing error into predictions of efficacious exposure in humans. Regulatory guidances have long required that impurities above some threshold be separately assessed for toxicity, and failure to produce a monodisperse linker at the outset merely defers that activity downstream, where isolation of a contaminant that is highly structurally similar to the active degraders can be an all but insurmountable chromatographic challenge. Thus, the effort expended to procure highly pure PEG reagents will be repaid downstream in both more precise SAR tables and more compressed timelines.
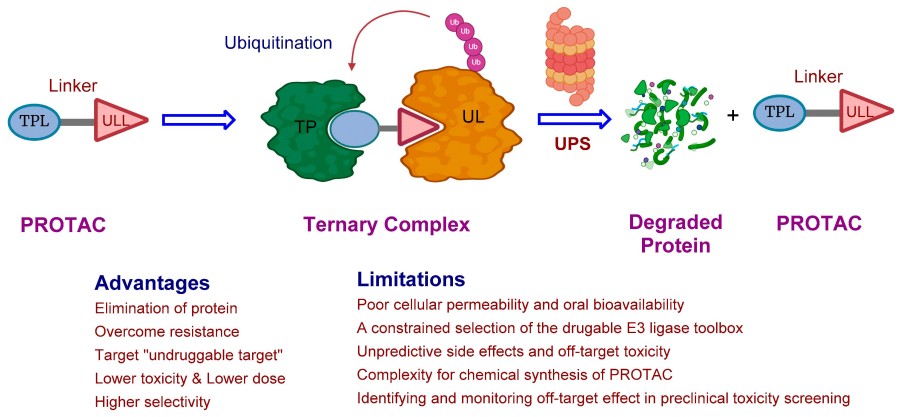 Fig. 1 Mechanism of PROTAC-mediated protein degradation.1,2
Fig. 1 Mechanism of PROTAC-mediated protein degradation.1,2
Avoiding Batch Variability
Batch-to-batch drift almost never manifests itself as lab scale synthetic disasters but as attrition in the details. A few minutes extra stirring time oxidizes a terminal alcohol, a few percent extra activating agent blocks the amine as an unreactive carbamate, a few ppm excess water inactivates the incoming electrophile and terminates chain elongation. These micro-heterogeneities within a linker library translate to slow and unnoticeable attrition in reproducibility: what was a crisp DC50 in last year's campaign is now inactive and requires resynthesis of the entire library under the misapprehension that something has changed in the biology when in fact the chemistry has been subtly compromised. Monodisperse PEG lots created via iterative chromatographic polishing and crystalline scavenging come with a certificate of analysis that explicitly states the oligomer length, the oxidation state of the end-groups and the metal ppm content. In doing so they effectively silence these hidden variables. Critically, they also allow chemists to build multi-gram stockpiles that stay analytically indistinguishable year after year so that one need not re-validate every step of the synthesis every time a new POI programme is started. The net effect is that one compresses decision-making cycles since one can be more confident that any observed change in cellular potency is a result of one's structural changes rather than silent linker degradation and so one can more rapidly converge degradation-tailored chemical space.
Ensuring Reliable SAR Studies
In the PROTAC field, the critical structure–activity relationships are described by a small free-energy window, within which one angstrom of spacer elongation is enough to destabilize the ternary complex into an unproductive conformation. If the library being screened already contains a statistically significant tail of mis-built oligomers, then the resulting DC50 no longer measures the intended variable, but rather reports on a population-weighted average of active, partially active, and inactive species. High-purity PEG linkers allow the researcher to circumvent this ambiguity by ensuring that every well in a 96-well matrix contains the same chemical species; any shift in potency can thus confidently be attributed to the intended warhead or E3 ligand change. The certainty is particularly important when the linker is itself the target of optimization – elongating from PEG4 to PEG6, for example, or inserting an intervening alkyne to rigidify the molecule. In this case, the expected change in potency is small, and the real signal can be easily drowned out by impurity noise, causing false negatives that prematurely end a potentially productive series. But purity also supports orthogonal readouts such as permeability, solubility, and plasma stability, all of which are inputs into multi-parameter optimization algorithms. If these inputs are polluted by linker heterogeneity, then the algorithm will hone in on false optima that later prove unsuitable when scaled up. In contrast, building a library on an analytically homogeneous spacer yields an SAR surface that remains predictive from milligram-scale plates to gram-scale tox preparations, safeguarding both scientific rigor and momentum in development.
PEG linkers are the unassuming framework that underpins nearly every bifunctional-molecule array in use today. They do not deliver potency on their own, but rather the only combination of properties that an HT campaign for degraders is likely to need: monodispersity, orthogonal chemistry, and an immediate polarity ladder. Teams can populate microtiter plates with pre-assembled E3–PEG synthons that vary only in length or end-group and translate a single POI warhead into a 200-member library overnight, certain that each well has been assigned the same analytical pedigree. The data matrix is self-normalizing: because the linker itself is chemically silent, any shift in degradation read-out can be unambiguously attributed to distance or vector angle rather than to batch impurities, a luxury that alkyl or peptide spacers (which suffer from solubility noise and proteolytic clipping) cannot offer. In short, PEG linkers do not just populate the library; they define its signal-to-noise floor, turning early discovery from a fishing expedition into a measurable optimization exercise.
High-Purity PEG4 Linkers - Ready for Your Next PROTAC Project
View the full catalog and specifications to explore our selection of high-purity PEG linkers optimized for targeted protein degradation.
Advantages in Early Discovery
At the hit-declaration stage, the principal uncertainty isn't if a POI is ligandable, it's if the two binding sites can be persuaded to adopt a catalytically productive orientation. PEG linkers answer this question with the least committing chemistry possible. The repeating ether oxygen atoms of the PEG backbone impose a hydrophilic yet non-ionic sheath that rescues lipophilic warheads from aggregation and allows assays to be run at concentrations high enough to detect weak ternary events without the optical interference of micellar turbidity. Because the oligomers are chemically identical from batch to batch, the investigator can array length from two to twelve glycol units in a single afternoon, generating a distance–response curve that immediately flags the geometric sweet spot; shorter chains that strain the complex register as flat lines, while over-long analogues reveal the classical hook plateau. The same plate can then be re-interrogated for permeability or microsomal stability without repurification, because the PEG backbone is essentially inert toward cytochrome oxidation or esterase cleavage. Perhaps most decisive is the compatibility with miniaturized parallel synthesis: the requisite hydroxyl or amine terminus reacts cleanly in 96-well format under aqueous or mildly alcoholic conditions, eliminating the need for anhydrous setups or precious-metal catalysts that constrain throughput. Consequently, a team can move from in-silico docking to cellular degradation data in less than a week, a cadence that would be impossible if each linker required a bespoke synthetic route. Early discovery, by definition, rewards speed over perfection; PEG linkers deliver speed while still furnishing data that remain valid once the programme transitions to lead optimization.
PEG Linkers and Scalability for Lead Optimization
With an optimal length identified, the identical PEG chemistry is amenable to scale up from mg-sized SAR arrays to multi gram tox lots without re-design of the synthetic route. The short chain ethylene glycol backbones are also single isomeric, so the impurity profile developed during micro-scale coupling can be expected to be similar to that under pilot-plant scale conditions, shortening the required analytical validation package that regulatory groups request. Furthermore, as the linker polarity can be tuned in discrete 44 Da steps, medicinal chemists can optimize lipophilic ligands that might otherwise exceed solubility thresholds, optimizing towards oral exposure without giving up the X-ray exit vector that serves as the starting point for ternary complex modelling. Finally, and perhaps most importantly, the synthetic manipulations that attach the POI warhead (usually amidation or reductive amination) are both high-yielding and compatible with a broad range of functional groups, so campaigns can run halogen scans, heterocycle swaps or stereochemical inversions while using the same PEG core. This degree of continuity not only preserves the validity of previous ADME data, but also avoids the otherwise inevitable re-qualification which slows many programmes that change spacer class mid-development. Even in the context of advanced delivery formulations, such as lipid nanoparticles, the hydrophilic PEG corona does not require further conjugation chemistry, easing tech-transfer from discovery to development. In short, the linker that was chosen to generate clean SAR on 96 well plates becomes the same chemical entity tested in rodent PK, dog tox and eventually GMP scale – a linearity in scalability that has made PEG oligomers into the silent, yet indispensable backbone of degrader libraries.
Manufacturing and Quality Standards
A final note on making bifunctional molecules is that the best of them come from rigorous manufacturing discipline at every linker batch that goes into libraries. A "high-purity" PEG plant, for example, must use identity/assay/impurity controls at each unit operation, not just at the end of the line as gatekeeper analytics. The process starts with raw-material qualification (each diol/amin-PEG comes with its own certificate tied to a validated synthesis) and is accompanied by in-process moisture/oxygen/metal monitoring, since ethylene glycol chains are extremely susceptible to oxidative scission and Lewis-acid-catalyzed ether cleavage. The release specs are only the tip of the iceberg. The real gold is in trending side-product profiles across dozens of pilot runs, plugging those data back into solvent choice/temperature ramp/quench sequence and then freezing the improved protocol in a master batch record that all future batches must follow to the letter. Done this way, the material doesn't just hit a spec sheet; it provides the same ternary-complex kinetics 3 years later, with no expensive SAR reversals due to one lot suddenly underperforming due to a stealth trace of PEG3 acid terminating the chain.
Analytical Testing (HPLC, LC-MS, NMR)
High-performance liquid chromatography (HPLC) continues to be the gold standard for demonstrating that the desired oligomer is the major component and that neighbouring homologues are below the level where they might interfere with the biological read-out. Reversed-phase methods using volatile buffers can be run such that fractions are collected and submitted directly to electrospray mass spectrometry, thus confirming not only molecular mass but also the presence or absence of di-acid, aldehyde or peroxide adducts that might otherwise escape UV detection. Quantitative NMR, usually against an internal standard of known stoichiometry, provides a orthogonal purity figure that is insensitive to UV response factors and therefore picks up UV-transparent impurities such as residual protecting groups or silicone oligomers leached from tubing. Two-dimensional NMR experiments (COSY, HSQC, HMBC) are run when a new synthetic route is introduced to ensure that regio-isomeric substitution or end-group epimerization has not taken place during scale-up. Taken together, the three techniques form a triangulated identity net: HPLC gives relative area, LC-MS assigns structural identity, and qNMR delivers absolute assay; only when all three converge is the batch released. This may seem like overkill, but the redundancy is not academic—regulatory reviewers increasingly request raw spectra alongside the summary table, and a well-documented analytical packet accelerates quality agreements with external CMOs because the receiving site can reproduce the method without re-development.
Compliance with International Guidelines
As small molecules, monodisperse PEG linkers are assessed for regulatory compliance under the same guidances as advanced intermediates. But the poly-ether backbone raises two other regulatory hot buttons: residual ethylene oxide oligomers and residual catalyst metals. Oligomer purification with a Class 2 solvent triggers control strategy for these solvents under ICH Q3C. And a risk assessment for elemental impurities as per Q3D could be called for, since the linker's sodium or potassium hydroxide base, used in the Williamson ether synthesis, could introduce unwanted contaminants from the metal salts. Linkers are, after all, active pharmaceutical ingredients destined for investigational new drugs, so auditors will expect to see validated cleaning methods to guard against cross-contamination, recorded change-control for any modification to column temperature or solvent ratio, and stability data that support the retest date on the label. The harmonized tripartite guideline goes even further in suggesting that suppliers provide a drug-master-file-style dossier, which enables end users to back-reference the linker's safety data without exposing proprietary process information. And as harmonized agencies clarify their thinking on nitrosamine or peroxide limits, the manufacturer will need to look back through her batch records and, if necessary, apply more stringent release criteria to finished goods long since delivered. A quality system that anticipates such regulatory drift–not just by capturing it in QMS documents, but by conducting periodic gap analyses and proactive method re-validations—will ensure that compliance is a living safeguard that benefits both the supplier and the drug developer.
Applications in Next-Gen PROTAC Pipelines
For both academic and translational efforts, PEG linkers have become de facto molecular "zip-ties" that turn high-affinity ligand pairs into catalytic degraders. PEG's utility is no longer limited to proof-of-concept cellular assays: late stage medicinal chemistry campaigns now consider the oligomer length as a tunable PK handle to rescue oral exposure, flatten the hook effect or enable intracellular click-assembly without compromising safety margins. As a result, the most frequently reported clinical candidates, be they directed against hematopoietic kinases, nuclear hormone receptors or aggregation prone neuroproteins, have retained a PEG4-to-PEG8 spine that has already cleared regulatory analytical scrutiny. In short, the linker is no longer a synthetic after-thought, but rather the architectural constant around which next-generation pipelines are being drafted.
Oncology and Neurodegeneration Research
PEG-linked degraders have enjoyed a rather seamless journey in oncology settings, from celltool compounds to first in human studies, due to the timely resolution of two historical bottlenecks: solubilization of otherwise aggressively lipophilic kinase warheads and adjustment of the dose window between tumor suppression and on-target hematologic toxicity. The modularity of short ethylene glycol linkers allowed swift interchange of recruiting ligases (CRBN, VHL or more recently IAPs) without the need to re-optimize the entire synthetic tree, which was found decisive when encountering resistance mutations that suddenly expanded the spatial distance between ligand-binding sites. Structural overlays suggest that PEG6 often populates a solvent exposed canyon between the target kinase and the E3 surface, where it acts as a dynamic hinge that allows the substrate lysine to sample multiple trajectories for ubiquitin transfer; shortening to PEG4 rigidly compresses the complex whereas lengthening to PEG8 occasionally allows an alternative hair-pin pose that can slow off-rate without penalizing kcat. Neurodegeneration programmes have leveraged the same toolkit for very different purposes: here, the challenge is not oncogenic over-expression but rather the entrapment of misfolded proteins that form robust protein–protein interfaces. PEG linkers afford enough conformational slack to let the degrader "reach over" bulky amyloidogenic segments and still place the E3 ligase within catalytic reach. Moreover, the hydrophilic cloak provided by three-to-six glycol units reduces non-specific membrane partitioning in the brain, which has been a liability that has sunk multiple central nervous system campaigns based on alkyl-only tethers. Preliminary rodent studies suggest that PEG-linked degraders can maintain unbound brain concentrations above the biochemical DC90 while avoiding the microvascular haemorrhage associated with overly lipophilic probes, positioning the same chemical scaffold as a translational bridge between malignancy and neuroprotection.
PEG Linkers in Clinical Trials
The emerging trend in the first investigational new drugs making it to patients is telling: the linker that survived rodent toxicology is nearly always the same PEG oligomer that entered the optimization funnel years before. Phase-I protocols published for haematologic B-cell malignancies report PEG4 or PEG6 as the stated intermediate, with the choice justified on the grounds of synthetic robustness and a lack of linker-specific adverse events at exposure multiples far above the expected therapeutic window. Agency reviewers have accepted monodisperse PEG chains as "well-established small-molecule fragments," freeing sponsors from the need to qualify residual ethylene oxide oligomers above ICH guidelines. The clinical experience, in turn, has fed back into discovery: medicinal chemists now treat the PEG length as a validated pharmacokinetic dial, lengthening when volume of distribution needs to be pushed into the extracellular space, shortening when central exposure must be limited. Perhaps more importantly, the public disclosure of safety data has de-risked follow-on programmes against entirely new gene products; investigators can enter the clinic with a PEG-linked degrader knowing that the spacer will not become the liability that derails development. The oligomeric ethylene glycol backbone has thus evolved from academic convenience to regulatory precedent, ensuring its continued dominance as PROTAC pipelines move beyond oncology into fibrosis, immunology and neurodegeneration.
Ordering Information
At BOC Sciences, we make it simple for research teams and pharmaceutical partners to source high-purity PEG linkers with full traceability and rapid turnaround. Whether you need a few grams for screening or multi-kilogram quantities for scale-up, our ordering process is streamlined, transparent, and globally accessible.
MOQ and Global Shipping
Our standard minimum order quantity (MOQ) for research-grade PEG linkers starts as low as 100 mg, allowing you to test and optimize linkers efficiently during early-stage SAR studies. For bulk or GMP-adjacent projects, we can scale production to multi-kilogram quantities with consistent purity and complete COA support.
We offer door-to-door global shipping through reliable logistics partners, including temperature-controlled and dry-ice options for sensitive materials. All shipments are tracked and packaged to ensure your products arrive in perfect condition.
Contact for Bulk and Custom Needs
Need something beyond standard PEG4, PEG6, or PEG8? Our custom synthesis service allows you to modify chain length, end-group functionality (amine, azide, alkyne, NHS, maleimide), or isotopic labeling to match your project's exact specifications. For bulk orders, multi-batch scheduling, or long-term supply agreements, our technical sales team will prepare a tailored quotation within 24 hours. We also provide supporting documentation, including analytical data, stability reports, and import/export assistance.
Reach out to our specialists today to request a quote or discuss your PEG linker requirements. We'll help you choose the optimal format, delivery schedule, and quality grade for your next-generation PROTAC program.
FAQs
1. Why is purity important for PEG linkers?
High-purity linkers minimize batch variability and improve reproducibility in PROTAC synthesis and screening.
2. What analytical tests verify linker quality?
Each batch undergoes HPLC, LC-MS, and NMR analysis with a detailed COA for identity and purity verification.
References
- Image retrieved from Figure 1 " Mechanism of PROTAC-mediated protein degradation.," Rej R K.; et al., used under [CC BY 4.0](https://creativecommons.org/licenses/by/4.0/). The original image was not modified.
- Rej R K, Allu S R, Roy J, et al. Orally bioavailable proteolysis-targeting chimeras: an innovative approach in the golden era of discovering small-molecule cancer drugs[J]. Pharmaceuticals, 2024, 17(4): 494. https://doi.org/10.3390/ph17040494.
- Kubryń N, Fijałkowski Ł, Nowaczyk J, et al. PROTAC Technology as a New Tool for Modern Pharmacotherapy[J]. Molecules, 2025, 30(10): 2123. https://doi.org/10.3390/molecules30102123.
- Rej R K, Allu S R, Roy J, et al. Orally bioavailable proteolysis-targeting chimeras: an innovative approach in the golden era of discovering small-molecule cancer drugs[J]. Pharmaceuticals, 2024, 17(4): 494. https://doi.org/10.3390/ph17040494.
- Zou Y, Ma D, Wang Y. The PROTAC technology in drug development[J]. Cell biochemistry and function, 2019, 37(1): 21-30. https://doi.org/10.1002/cbf.3369.

 Fig. 1 Mechanism of PROTAC-mediated protein degradation.1,2
Fig. 1 Mechanism of PROTAC-mediated protein degradation.1,2