Optimizing the linker was once considered a secondary step but is now becoming an integral part of PROTAC design, since the linker directly determines whether a PROTAC will become a druggable compound or remain a chemical novelty. It must meet a number of geometric, electronic and pharmacokinetic requirements: it must be able to span the distance between two proteins without clashing with their surfaces, have sufficient polarity to be soluble but not so much that it will be impermeable and be sufficiently stable to the first-pass effect to allow for measurable levels of degradation. Recent publications have highlighted how a single-atom change, such as replacing a phenyl with a pyridyl, can reverse isoform specificity or dramatically improve oral bioavailability, explaining the shift in the PROTAC field to optimizing the linker choice in parallel with the ligands, rather than as a final step.
Why Linker Choice Matters?
The linker stands out as the sole moiety exposed to bulk solvent while also contacting both the ligase surface and target surface resulting in its chemical nature affecting every downstream property. A properly matched tether enables ternary complex pre-organization to overcome binary affinity deficits while a misfit tether causes program failure regardless of nanomolar binding levels. Understanding how length, flexibility, polarity and metabolic soft-spots interplay is therefore as critical as warhead design itself.
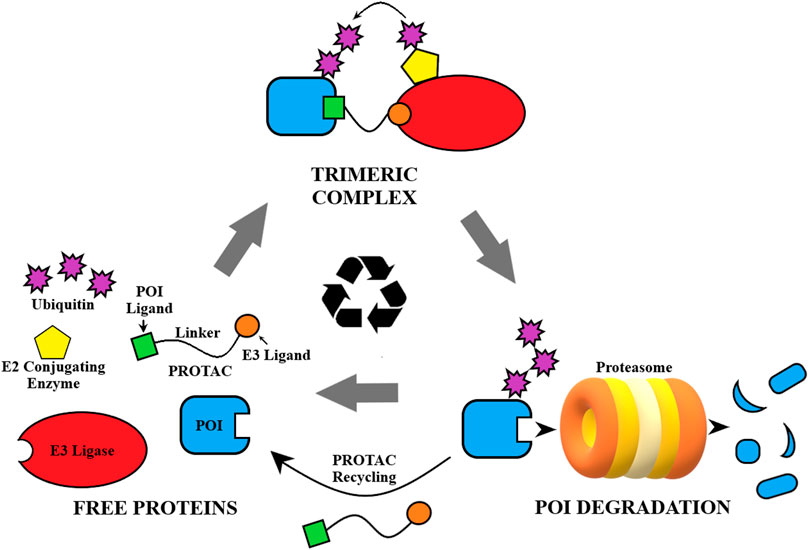 Fig. 1 General depiction of the mechanism of action of PROTACs.1,5
Fig. 1 General depiction of the mechanism of action of PROTACs.1,5
Impact on Ternary Complex Stability
Stability of the ternary complex is determined by how well the linker places the substrate lysine in catalytic proximity without entropic, steric or electrostatic penalty. An insufficient tether collapses the two proteins into the same volume, resulting in repulsive contacts and increasing the free energy of association. Overextended tethers are associated with an entropic cost of folding and the increased possibility of non-productive binding modes in which the lysine is oriented away from E2-loaded ubiquitin. Crystallographic surveys therefore point to an aspect ratio which is neither fully collapsed nor fully extended with a linker that acts as a molecular ruler by placing the reactive amide at a separation amenable to nucleophilic attack. In addition to length, regiochemistry and the presence of heteroatoms also tune stability. Inclusion of a pyridyl nitrogen that is a potential acceptor for a hydrogen bond donated by a backbone NH results in a transient staple which can increase residence time without the same increase in molecular weight as might be expected for a chain extender. Molecular dynamics trajectories of heteroaryl rich linkers sample fewer rotational clusters and stay longer in the productive basin, leading to faster ubiquitin transfer and more complete degradation with lower exposures. Therefore, linker choice serves as a conformational catalyst to translate high affinity binding to sustained catalytic turnover.
Influence on PK/PD and Bioavailability
In addition to facilitating binding, the linker also establishes key whole-molecule properties that influence absorption, distribution and clearance. Alkyl chains increase lipophilicity and passive permeability, but also increase volume of distribution and lead to ω-oxidation, both features that are associated with reduced half-life and the production of reactive metabolites. Polymethylene poly(ethylene glycol) (PEG) oligomers increase solubility, but also molecular weight and polar surface area, pushing the overall construct outside of the oral absorption window unless rigid cap groups are included to increase the aspect ratio. Heteroaryl or nitrogen-rich moieties represent a compromise, in that they bring in directional lone pairs that increase kinetic solubility without adding permanent charge, and the electron-deficient ring is less prone to oxidative metabolism than a phenyl ring. Basic nitrogens can be protonated within acidic endosomes, for example, to enhance membrane escape, but remain predominantly uncharged in the cytosol, expanding therapeutic index without the risk of tissue sequestration that can come with permanent charge. Finally, the linker can tune tissue selectivity: a morpholine–urea hybrid that targets a protein within the central nervous system (CNS), for example, needs to balance polarity and logP to enable blood–brain barrier penetration, while a peripheral target degrader may gain selectivity advantages from quaternization to limit distribution. Taken together, these considerations show that linker design cannot be decoupled from PK/PD optimization; a spacer that fixes ternary complex geometry must also meet solubility, permeability and metabolic requirements if the PROTAC is to make the journey from biochemical hit to orally active drug.
Expert Insights by Linker Type
The different linker families are characterized by a unique thermodynamic and pharmacologic profile. PEG provides entropic free space and hydrophilicity, alkyl groups provide low molecular weight hydrophobicity to aid in cell membrane permeation, aromatic groups provide conformational rigidity and the ability to form π-π interactions, and nitrogen-containing heterocycles allow for directional hydrogen bond donor-acceptor (HBD/HBA) capability as well as pH-sensitive ionization. Experienced medicinal chemists often consider the linker families as orthogonal "dials" to tune rather than binary decisions, mixing the desired properties until the half-life no longer increases or unwanted PK properties are affected.
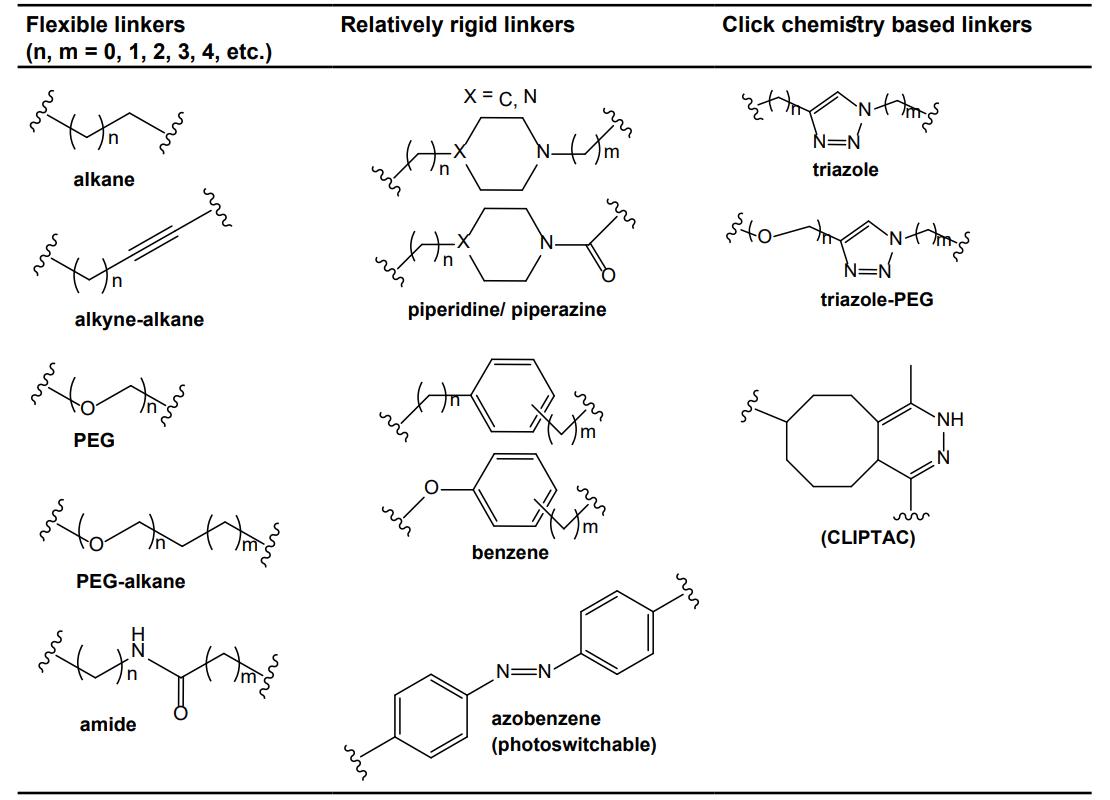 Fig. 2 The common types of linkers used for the design of PROTACs.2,5
Fig. 2 The common types of linkers used for the design of PROTACs.2,5
PEG is the standard hydrophilic spacer since it encodes a degree of conformational freedom and aqueous solubility in a chemically inert manner. The repeating ether oxygen serves as a dynamic hydration shell that quenches aggregation and shields hydrophobic patches on the conjugate, reducing non-specific binding and prolonging circulatory residence time without increasing molecular charge. Conformationally, PEG is similar to a molecular worm: fast rotation around the C–O bonds enable the chain to sample extended and collapsed states, increasing the chance of both the warhead and ligase ligand simultaneously finding their pockets during a single diffusional encounter. Multiconformational ability is a much sought after characteristic during hit-to-lead optimization, when the ideal separation distance is not yet known. The addition or removal of a single ethylene-glycol unit is an easy way to add a defined length increment, without redesigning the synthetic route. Problems start to arise, however, when the oligomer reaches a length of six to eight units: Larger molecular weight and greater polar surface area drive the build beyond oral absorption performance limits and subsequent ether breakdown leads to lipophilic fragments which cell assays struggle to detect. Contemporary practise therefore restricts PEG to short, monodisperse lengths (n = 2–6) or marries it to a rigid heteroaryl cap that restores aspect ratio without adding more glycol mass. Despite these caveats, PEG remains the gold-standard solubility module for injectable or early-cell-assay PROTACs because its physicochemical signature is predictable and its terminal chemistry (azide, amine, halide) can be installed overnight under aqueous conditions.
Straight-chain alkyl tethers remain a common scaffold class in orally active degraders as they provide hydrophobic span for relatively low atomic overhead. Every methylene unit encodes roughly 2.5 Å of distance, allowing for fine-tuned length scanning without the need for computational modelling, and the complete lack of polar atoms ensures that molecular weight and logP remain low - properties which correlate to higher membrane permeability and oral exposure. Their flexibility is both an advantage and a liability: it allows the chain to collapse into a compact hair-pin conformation, which may alleviate the entropic penalty associated with ternary complex assembly, but also makes the linker more susceptible to ω-oxidation. Cytochrome P450s abstract benzylic or allylic hydrogens, forming alcohols that can be further oxidized to carboxylic acids; these more polar metabolites are frequently affinity-retaining, which muddies in-vivo interpretation. To reduce the likelihood of this process, modern alkyl linkers are typically terminated by electron-withdrawing amides or capped with short rigid motifs (alkyne, cyclopropane) that protect against oxidative attack while preserving the low-mass advantage. Branching is typically avoided as it increases logP disproportionately, but a single γ-methyl group can protect adjacent C–H bonds without significantly perturbing aspect ratio. In sum, short alkyl fragments (C4–C10) remain the permeability module of choice when exposure rather than potency limits the therapeutic index.
Aromatic rings morph the linker from a passive strap to a conformationally locked rod whose length is determined by π-bond geometry rather than rotatable bonds. Phenyl, biphenyl or phenyl-ethynyl units impose a planar, hydrophobic spine that pre-organizes the PROTAC into an extended conformation, often steepening the degradation curve by lowering the entropic cost of ternary complex assembly. The delocalized π-cloud participates in edge-to-face or offset stacking with phenylalanine, tyrosine or histidine side chains which frequently line the target–ligase interface, providing enthalpic glue that supplements the binary affinities of the terminal ligands. On the metabolic side, the electron-rich ring is also less prone to oxidative cleavage than alkyl chains; hydroxylation prefers para positions and can be sterically blocked by small fluorine substituents without perturbing π-stacking. The main limitation is on solubility: consecutive aryl rings increase crystal lattice energy and can cause colloidal aggregation even at micromolar concentrations. This issue is typically bypassed by inserting one heteroatom (ether, sulfone) or by capping the aromatic stack with a basic nitrogen, both of which disrupt symmetrical packing but retain rigidity. Synthetic accessibility is no longer a barrier: brominated or boronated aromatics are commodity reagents that couple under aqueous Suzuki conditions, allowing rigidification to be explored in parallel rather than in series.
Heteroaryl and saturated nitrogenous rings combine the shape persistence of aromatic rings with the polarity and hydrogen-bond directionality offered by lone pairs. Pyridine, pyrimidine, triazole or imidazole motifs bring an sp² nitrogen with a lone pair in an orientation that can accept a hydrogen bond from backbone NH groups, thus stapling the nascent ternary complex in place without contributing to molecular weight. Choice of regiochemistry is a further dial, with 3-pyridyl leaving the lone pair in a protected position, or 4-pyridyl placing the dipole in-line with the linker axis for situations where the interface could use an electrostatic zipper. Saturated equivalents (piperazine, morpholine, azepane) can contribute pH-tunable basicity, useful for endosomal escape, but generally neutral in the cytosol for better oral exposure. Nitrogen-containing rings are also more metabolically robust than phenyl, as the electron-deficient ring offers less favourable targets for oxidative attack (in practice N-oxidation will be the main pathway, generating polar metabolites which are readily excreted). Synthetic orthogonality is also high, with azides, boronic acids and halogenated heteroarenes all coupling under mild and functional-group-tolerant conditions, enabling rapid iteration of structures that is more difficult to achieve with highly substituted alkyl chains. For these reasons nitrogen-containing motifs are rapidly becoming the most popular linker category in modern PROTAC research.
Common Pitfalls in Linker Design
Linker design is an exercise in compromise where the line between "just right" and "disastrously wrong" is a difference of a single atom. The most common mis-steps (over-polarization and over-rigidity) are seductive because each one perfectly solves one problem while quietly incubating another. Learning to recognize the early warning signs (precipitates in serum, shallow degradation curves or sudden loss of cellular activity) allows teams to back-pedal before the entire programme is committed to an irreversible scaffold.
Over-Polarization and Poor Permeability
One temptation is to repeat the insertion of heteroatoms, sulfones or PEG oligomers until aggregation no longer appears and the aqueous solubility curve plateaus. The side-effect, however, is a precipitous fall in passive membrane permeability: the polar surface area breaches an implicit limit beyond which paracellular transport is no longer possible and transcellular flux is rate-limited by tight-junction restriction. Intestinal epithelium or blood–brain barrier effectively become an in-line filter, stripping the PROTAC out of the absorption pool before it ever reaches the intracellular space where degradation occurs. Hyperpolarization also increases efflux liability; P-glycoprotein recognizes extended, oxygen-rich backbones and pumps them actively back to the lumen, countering oral exposure even with high dissolution. A second, subtler effect is lysosomal trapping: after the molecule is protonated in acidic endosomes, the cationic species is kinetically trapped, which increases total tissue levels without a rise in free cytosolic concentration - an artefact which can be mistaken for target engagement. Lastly, too much polarity causes the compound to get filtered out in the kidney before being metabolized, therefore having a very short half-life and necessitating more frequent or higher dosing. Rescue strategies generally involve "polarity weaning": an ether oxygen is substituted by an alkyne, or a terminal morpholine is swapped for a piperidine, changes which preserve solubility at acidic pH while restoring enough logP for passive trans-membrane diffusion. The takeaway is that solubility and permeability must be optimized in tandem; if one only chases the former it almost guarantees a downstream PK failure.
Over-Rigidity and Loss of Activity
The obvious advantage of rigidification, be it by long biphenyls, alkyne rods or fused heteroarenes, is that the entropic penalty for ternary complex formation has already been paid upon synthesis, so residence times are increased and degradation curves made steeper. However, beyond a certain point of rigidity, the same linker can become a mechanical straightjacket. Proteins are not static objects, the target-ligase interface often breathes by several ångströms over the course of the catalytic cycle. An overly stiff linker does not allow for this adaptive motion, effectively forcing the lysine sidechain to reside just outside the ubiquitin-loading radius, even when binary affinities are high. Crystal structures have often reported intact ternary complexes with no signs of ubiquitination, an effect that could be linked back to sub-ångström misalignment which the linker is unable to compensate for. Too much rigidity also often manifests in solid state, aggregation-prone behavior: planar, π-stacked rods tend to crystallize as needles that have slow solubility, and can precipitate colloidal aggregates at micromolar concentrations. Metabolically, the electron-rich aromatic highway provides an attractive scaffold for CYP oxidation, leading to phenolic metabolites that maintain affinity but have different clearance pathways. Finally, the more rings that have to be connected, the more synthetic steps become necessary, with each ring requiring a cross-coupling step under harsh, oxygen-free conditions that increase cost and slow down iterative cycles. The common solution is to "soften" one hinge, often by inserting a single saturated carbon, a sulfone or a short PEG unit, to provide just a few extra degrees of rotational freedom, without unduly losing the pre-organization advantage. The takeaway is that rigidity is not an absolute property, and must be judiciously dosed, the goal being to guide, rather than immobilize, the protein partners.
How We Support Your Research?
At BOC Sciences, we don't just supply linkers-we partner with your team to ensure every molecule you design performs at its highest potential. From linker selection to custom synthesis and scale-up support, our chemists provide expert guidance and fast, reliable solutions for all stages of PROTAC development.
Choosing the optimal linker can be complex-balancing solubility, rigidity, permeability, and protein–ligase geometry requires both chemical insight and hands-on experience. Our free consultation service connects you directly with experienced PROTAC chemists who can:
- Analyze your current target and E3 ligase pairing.
- Recommend linker types (PEG, alkyl, aromatic, or heteroaryl) based on desired pharmacokinetic and structural profiles.
- Suggest optimized linker lengths and functional groups for improved ternary complex formation.
- Review experimental data to guide iterative design and SAR refinement.
Each consultation is confidential and customized to your research program—no generic answers, just focused scientific support that helps you make faster, smarter design decisions.
Custom Synthesis and Bulk Ordering
Our synthesis capabilities are built for flexibility. Whether you need one-off prototypes for SAR studies or multi-kilogram batches for preclinical work, we deliver quality and consistency at every scale. Our services include:
- Custom linker synthesis with tailored length, polarity, and end-group modifications.
- Hybrid linker designs, such as PEG-alkyl or PEG-aromatic combinations.
- Bulk manufacturing for recurring supply and large research programs.
- Full analytical validation (HPLC, LC-MS, NMR) and COA documentation included with every shipment.
With short lead times and global delivery, we make it easy to integrate our linkers into your existing research pipeline-quickly and confidently.
Partner with Our Experts for Reliable PROTAC Linker Solutions
Selecting the right linker is one of the most decisive steps in PROTAC design, influencing everything from molecular stability to cell permeability and degradation efficiency. Working with an experienced, reliable supplier ensures your research stays both innovative and reproducible. At BOC Sciences, we combine scientific expertise, precision chemistry, and customer-centered support to provide the linkers and guidance you need to succeed. Whether you're optimizing a novel degrader or scaling up a validated candidate, our team is ready to help.
Schedule your free consultation today or request a custom synthesis quote to accelerate your next PROTAC project. Partner with a chemistry team that understands your science-and helps you transform it into results.
High-Purity PROTAC Linkers - Ready for Your Next PROTAC Project
View the full catalog and specifications to explore our selection of high-purity linkers optimized for targeted protein degradation.
FAQs
1. Why is linker choice so critical in PROTAC design?
It determines ternary complex formation, degradation efficiency, and pharmacokinetics.
2. Do you provide scientific support?
Yes, our experts offer free consultations to review your design and recommend suitable linkers.
3. Can I request custom linkers or scale-up production?
Absolutely—custom synthesis and multi-kilogram scale-up services are available on demand.
4. How can I get started?
Simply contact our technical team for a consultation or request a quote online.
References
- Burke M R, Smith A R, Zheng G. Overcoming cancer drug resistance utilizing PROTAC technology[J]. Frontiers in Cell and Developmental Biology, 2022, 10: 872729. https://doi.org/10.3389/fcell.2022.872729.
- Zagidullin A, Milyukov V, Rizvanov A, et al. Novel approaches for the rational design of PROTAC linkers[J]. Exploration of Targeted Anti-tumor Therapy, 2020, 1(5): 381. https://doi.org/10.37349/etat.2020.00023.
- Kubryń N, Fijałkowski Ł, Nowaczyk J, et al. PROTAC Technology as a New Tool for Modern Pharmacotherapy[J]. Molecules, 2025, 30(10): 2123. https://doi.org/10.3390/molecules30102123.
- Zattoni J, Vottero P, Carena G, et al. A comprehensive primer and review of PROTACs and their In Silico design[J]. Computer Methods and Programs in Biomedicine, 2025: 108687. https://doi.org/10.1016/j.cmpb.2025.108687.
- Distributed under Open Access license CC BY 4.0, without modification.

 Fig. 1 General depiction of the mechanism of action of PROTACs.1,5
Fig. 1 General depiction of the mechanism of action of PROTACs.1,5 Fig. 2 The common types of linkers used for the design of PROTACs.2,5
Fig. 2 The common types of linkers used for the design of PROTACs.2,5