Polyethylene-glycol (PEG) linkers serve as entropic-rich, hydrophilic tethers that increase separation distance and reduce aggregation in PROTAC chimeras. The repeating ether backbone creates a dynamic hydration shell, enabling the conjugate to sample an ensemble of extended or collapsed conformations without the enthalpic penalty of desolvating a hydrocarbon chain. Short PEG oligomers have rescued otherwise insoluble degraders, across a number of recent oncology and neurodegeneration programmes, steepening dose–response curves and enabling intravenous or oral delivery without reformulation.
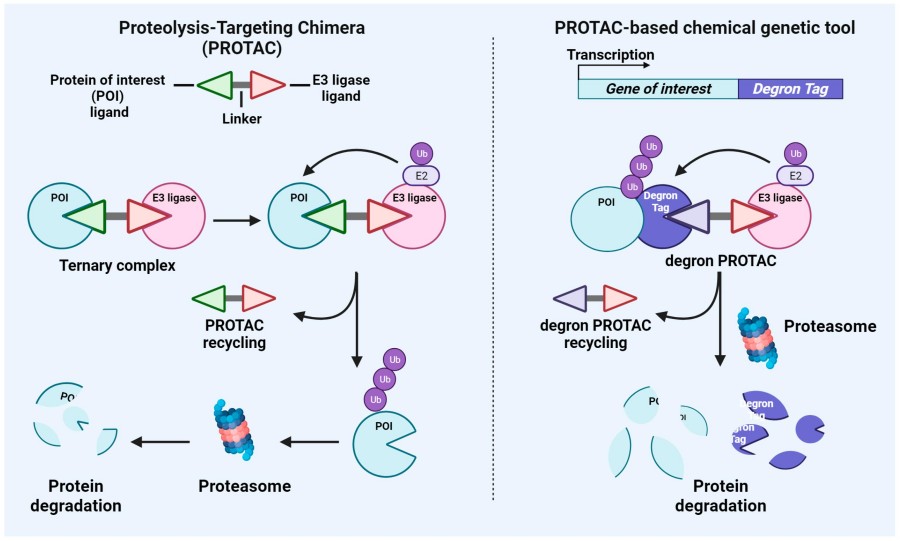 Fig. 1 Schematic representation of the mechanisms of action of PROTAC and PROTAC-based genetic tools.1,5
Fig. 1 Schematic representation of the mechanisms of action of PROTAC and PROTAC-based genetic tools.1,5
Introduction to PEG Linkers
PEG linkers were initially repurposed from protein-modification chemistry where they were used to shield immunogenic epitopes and to extend half-life. In PROTACs, PEG has a more elaborate function: to act as conformational shock absorbers that allow the warhead and E3-recruiting ligand to independently arrive at their most favorable mutual orientation while remaining in a fully hydrated state. The upcoming sections will decompose the molecular underpinnings of this versatility and clarify why going too long with PEG can reverse the advantage.
What Are PEG Linkers?
PEG linkers are oligomers of repeating ethylene-oxide units (–CH2–CH2–O–) that are capped by a chemically addressable handle (azide, amine, maleimide or halide). The ether oxygen is both a hydrogen-bond acceptor and a pivot of low-barrier rotation; this "worm-like" flexibility allows the chain to extend, coil or kink in response to the spatial demands of the ternary complex. Because each repeating unit adds approximately 3.5 Å of contour length, a chemist can probe separation distances in discrete, predictable increments without redesigning the synthetic route. Preparative HPLC can routinely isolate monodisperse batches (n = 2–8). This means that the conjugate will have a fixed hydrodynamic radius, an important metric for reproducible pharmacokinetics. The ether backbone is similarly essentially transparent to cellular enzymes: it is resistant to oxidative cleavage that would liberate the payload because only high-potential metal-oxo species, which are rare in the cytosol, can cleave ethers. As a result, it is unreactive until an explicit trigger (ester, disulfide or photocage) is engineered into the chain. The polar, uncharged nature of PEG also means that it raises polar surface area without adding a permanent charge. This balance preserves membrane permeability while discouraging off-target adsorption to serum proteins or plastic labware.
Why PEG Matters in PROTAC Research?
In practice, PROTAC campaigns find PEG playing three roles at once. It serves first as a solubility buffer: a hydration shell sequesters hydrophobic ligands and averts the colloidal aggregation which can be especially pernicious for extended aromatic rods. Second, it acts as an entropic spring—occupying both extended and collapsed conformers, the linker raises the odds that the warhead and E3 ligand will simultaneously occupy their respective pockets during a single diffusional encounter, an effect that can steepen cellular DC₅₀ values without affecting binary affinity. Third, PEG provides a metabolic firewall: lacking benzylic C–H bonds, it removes the prime target of microsomal hydroxylation, extending intracellular half-life and enabling lower dosing. Caveats arise when the oligomer is stretched beyond six to eight units, though: molecular weight and polar surface area swell, ejecting the construct from the oral absorption window, and the same hydration shell which can avert aggregation can also form a steric shield, hindering membrane translocation. Modern practice thus considers PEG as a "Goldilocks" module—short enough to maintain permeability, long enough to rescue solubility, and capped with rigid or heteroaryl moieties which restore aspect ratio without additional glycol mass. Used sparingly, PEG remains the most reliable path to a water-soluble, metabolically silent PROTAC that behaves predictably in cellular and animal assays.
Key Benefits of PEG Linkers
PEG linkers possess a unique pairing of aqueous liberality and conformational complacency: the repeating ether oxygens drape a hydration blanket that solubilizes otherwise insoluble payloads, while the near-frictionless C–O bonds allow the two ends of a PROTAC to sample a hemisphere of orientations within the ternary complex. These two benefits—solubility and flexibility—feed directly into shorter optimization cycles, steeper degradation slopes and fewer excipients in the final formulation.
Improving Water Solubility
PEG oligomers are molecular surfactants that don't aggregate. The ether oxygen binds two vicinal waters to form a dynamic hydration shell whose effective radius exceeds the covalent contour length of the chain. The shell's hydrodynamic volume enlarges it enough to slow renal ultrafiltration without exceeding the Rule-of-5 envelope in molecular weight. The hydration layer is bound by hydrogen bonds, not permanent charge, so the whole construct is electrically neutral, unlike permanently protonated linkers whose fate is sealed by cation-transporter-mediated clearance. A subtler benefit is hydration of the hydrophobic protein surface: ordered waters are displaced when the PEGylated PROTAC binds its site, and the gain in translational entropy provides an additional driving force for association that can be mistaken for "affinity maturation". Finally, the hydration shell is a colloidal buffer: salts, surfactants or serum proteins that would otherwise trigger aggregation are instead intercepted by the PEG corona, giving assay read-outs that mirror true pharmacology rather than precipitation artefacts.
Enhancing Flexibility and Conformational Freedom
The C–O bond is shorter and more rotatable than a C–C bond, making PEG act as a molecular universal joint: it can transmit force without enforcing a fixed angle. Within the ternary complex, this generosity allows the warhead and the E3 ligand to sample microsecond-scale breathing motions of the protein interface, increasing the probability that the catalytic lysine will transiently occupy the correct radius for ubiquitin transfer. This same freedom also provides protection from mechanical stress: should target and ligase happen to drift apart by a few tenths of an ångström, the PEG coil can stretch rather than relay strain into the protein–protein interface, minimizing the formation of non-productive encounter complexes that would sequester the PROTAC in a dead-end trimer. Entropically, the chain incurs a cost, but this penalty is at least partially compensated by the low rotational barrier of the ether linkage; inspection of molecular-dynamics snapshots reveals that even a short PEG segment adopts dozens of low-energy conformers within the binding lifetime, effectively diluting the entropic cost over many microstates rather than a single high-energy pose.
Supporting SAR Studies
Since PEG chains are synthesised by iterative ether formation, length can be increased or decreased in single repeat units without changing the terminal functional groups. This level of granularity converts linker optimisation from a leap into a titration: chemists can synthesise a homologous series spanning three to twelve ethylene oxides, screen for cellular stability, and identify the minimum length that retains cooperative complex formation. The same monodispersity makes analytical validation easier—each oligomer produces a single LC–MS peak, without the polydispersity envelopes that complicate quantitative PK. Finally, the chemical inertness of the ether backbone localises metabolic liabilities to the warhead and ligase ligand, allowing linker length to be tuned without adding new oxidative soft spots; this orthogonality collapses weeks of multiparameter SAR into a single, well-behaved dimension.
Common PEG Linker Variants Used in PROTACs
Monodisperse PEG oligomers, most commonly PEG2, PEG4 and PEG6, are now the de facto standard for hydrophilic linkers. This is in large part because of their synthetic accessibility and relatively predictable physicochemical properties. The addition of an ethylene-glycol unit contributes about 3.5 Å of contour length and 44 Da of molecular mass, giving the chemist the flexibility to rationally tune the aspect ratio without redesigning the synthetic route from scratch. The frequent observation from comparative crystallography and cellular wash-out studies is that PEG2 provides the sweet spot for ternary-complex stability, whereas PEG6 and higher homologues are often saddled with entropic penalties and poor membrane permeability that can outweigh their intrinsic solubility. The above structure–activity rules, in concert with the gram-scale, chromatography-free availability of such building blocks, are also why short PEG homologues are so often used in early PROTAC discovery efforts.
PEG2 vs PEG4 vs PEG6 – Length Considerations
PEG2 confers the smallest hydration shell: two ether oxygens are sufficient to quench aggregation, but the backbone is still shorter than most ligand–ligase distances, making it well-suited to "tight" geometries where the binding pockets are situated almost face-to-face. Cellular uptake is still high as the increase in polar surface area is moderate and the molecule can still adopt a low-polarity conformer when traversing the membrane. PEG4 clusters around the sweet-spot for many published degraders—four repeats provide enough flexibility to mitigate minor steric mismatches, while the contour length remains within the range that facilitates cooperative ubiquitin transfer. The hydration shell is now significant, so solubility is rarely a concern, but the hydrodynamic radius has not yet passed a threshold that would compromise renal clearance. PEG6 starts to show signs of diminishing returns: six ether units impart sufficient conformational entropy that the effective molarity of productive ternary poses decreases, and the larger hydrated volume diminishes permeability across tight epithelia. Nevertheless, PEG6 remains well-suited when the target and E3 sites are separated by an oblique path that requires an elongated reach, or when the warhead itself is highly hydrophobic and demands an inflated solubility enhancement. In practice, most teams start optimisation at PEG4 and titrate outwards or inwards by single repeats, a process streamlined by the commercial availability of monodisperse PEG2, PEG4 and PEG6 diacids or diamines with identical end-group chemistry.
Synthetic Accessibility and Scalability
Access to monodisperse PEG linkers is achieved by iterative mesylation–displacement cycles from readily accessible tri- or tetra-ethylene glycol precursors. This approach bypasses the polydispersity issues arising from classical polymerisation, and each elongation cycle can be conducted in either water or alcoholic media without recourse to anhydrous conditions. In addition, direct crystallisation of the intermediate mesylate removes transition-metal residues that would poison downstream bioconjugations. As the molecular weight gain per repeat is low (≈ 44 Da), scale-up is linear: the same molar excess of base that provides gram quantities in the laboratory can be translated to kilogram campaigns without the need to re-optimize stoichiometry or solvent volumes. PEG linkers are also favoured by regulatory agencies for first-in-human studies since the metabolic fate is well-understood (ω-hydroxylation followed by glucuronidation), leading to metabolites that are already present in the food chain as cosmetic and pharmaceutical excipients. Finally, the crystallinity of short PEG diacids simplifies quality control: a single recrystallisation routinely affords material of > 98 % purity by qNMR, compressing release testing to a single assay rather than the multi-method packages required for more complex heteroaryl scaffolds.
Applications in Drug Discovery
PEG linkers have evolved from a tool of protein-modification chemistry to a central PROTAC scaffold in its own right. This is due in no small part to their propensity to rescue solubility, fine-tune flexibility and drive SAR iterations, all without requiring proprietary synthetic routes. Indeed, representative oncology programmes suggest that swapping a rigid biphenyl tether for a short PEG2 chain is often sufficient to rescue oral exposure with little to no loss in nanomolar degradation efficacy, and meanwhile emerging CNS-directed campaigns have leveraged PEGylated degraders to enhance brain penetration by reducing aggregate-associated efflux. Successes such as these, in conjunction with the commercial availability of monodisperse, GMP-compatible building blocks, are why PEG has become the default hydrophilic module in pre-clinical tool compounds as well as first-in-human candidates.
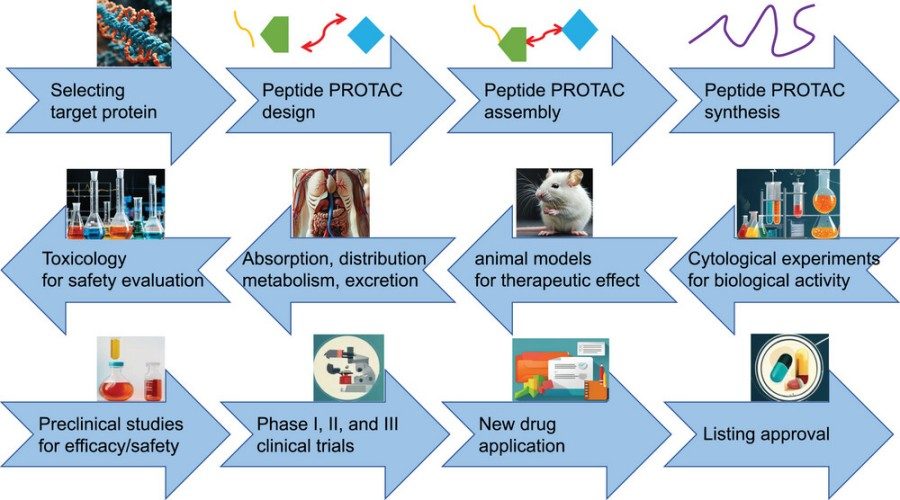 Fig. 2 Flowchart of the drug development process of peptide PROTAC.2,5
Fig. 2 Flowchart of the drug development process of peptide PROTAC.2,5
Case Studies from Literature
One illustrative example is a BET-family degrader targeting BRD4. Initial hits based on a purely aromatic linker had picomolar biochemical potency but showed no measurable degradation in whole blood due to precipitation of the construct at micromolar concentrations. Solubility was restored through replacement of the central biphenyl by a PEG4 unit, with no increase in span; the optimized PROTAC showed a magnitude improvement in cellular DC50 and, critically, an exposure–response curve that was linear across mouse, rat and dog species—an effect credited to the hydration shell that followed the PEG chain during absorption and distribution processes. Another example is an EGFR degrader intended to tackle osimertinib-resistant triple mutants. In this case, a PEG2 unit was placed between the anilinoquinazoline warhead and the VHL ligand; the short and flexible tether allowed the mutant kinase to assume the slightly twisted conformation necessary for cooperative complex formation, restoring degradation potency while preserving oral bioavailability. Perhaps the most elegant example is a dual-degrader that targets MDM2 and GSPT1 concurrently. By threading a PEG6 linker through a stapled peptide architecture, the authors constructed a branched PROTAC whose two arms were free to pivot independently; the resulting molecule retained nanomolar activity against both proteins and showed sustained tumour regression in xenograft models, demonstrating how PEG length can be modulated to accommodate multi-target approaches without compromising physicochemical properties.
Why PEG Is Widely Adopted in Clinical PROTAC Candidates?
In addition to its performance at the bench-scale, PEG linkers also meet a number of translational criteria that has hastened their move into the clinic. Monodisperse oligomers (n = 2–6) are now available under GMP-like manufacturing conditions and with validated impurity profiles, which mitigates the batch-to-batch heterogeneity that is a hallmark of polydisperse starting materials. The synthetic method is commonly a two-step amide coupling, which uses aqueous solvents and generally avoids the use of cryogenic or heavy-metal catalysts, to meet the green-chemistry guidelines set forth by regulators. In terms of PK, short PEG chains will increase kinetic solubility while minimally contributing to molecular weight, so it will not exceed the window for oral exposure. In addition, the ether backbone of PEG is less prone to oxidative cleavage compared to alkyl tethers, which endows the linker with the metabolic stability necessary for once-daily dosing. Most importantly, the hydration shell created by the PEG linker mitigates nonspecific binding to both plasma proteins and plastic labware, which helps to ensure that potency values that are measured in biochemical assays are faithfully recapitulated in cellular and animal assays. It is these advantages, which encompass manufacturing, PK, and reproducibility, that explain why PEG linkers are now incorporated in a number of clinical-stage PROTACs, and set a bar for newer hydrophilic technologies to meet or exceed.
How to Source High-Quality PEG Linkers?
Selecting a dependable supplier for PEG linkers is crucial for achieving consistent results in PROTAC design and drug discovery. At BOC Sciences, we manufacture and supply a complete range of PEG linkers-from PEG2 to PEG12-engineered for superior solubility, flexibility, and reproducibility. Every product undergoes rigorous analytical validation to ensure research-grade performance across all applications.
Purity Standards (NMR, LC-MS Confirmed)
All PEG linkers in our catalog are verified through advanced analytical methods:
- HPLC ensures accurate purity quantification and impurity detection.
- NMR (1H and 13C) confirms structural integrity and composition.
- LC-MS validates molecular identity and batch consistency.
- Each batch includes a Certificate of Analysis (COA) and detailed QC data, guaranteeing reproducibility and traceability.
These strict quality standards give researchers full confidence that every linker performs consistently across synthesis runs and biological assays.
Ordering and Customization Options
We offer flexible sourcing options to meet diverse research and manufacturing needs:
- Ready-to-ship linkers available from 100 mg to multi-gram quantities.
- Bulk supply and multi-kilogram production for preclinical and scale-up studies.
- Custom synthesis of PEG variants with functional groups such as amine, azide, alkyne, NHS, or maleimide.
Our technical chemists provide consultative support to help you select the optimal PEG length and end-group configuration for your target-ligase system, ensuring efficient ternary complex formation and high biological performance.
Partner with Us for Reliable High-Purity PEG Linkers
PEG linkers remain the cornerstone of modern PROTAC chemistry, providing the flexibility and solubility essential for effective target degradation. Partnering with a trusted supplier ensures your projects move from design to discovery with precision and speed. At BOC Sciences, we combine analytical excellence, scalable synthesis, and global distribution to deliver high-purity PEG linkers you can rely on-ready for immediate use or fully customized to your specifications.
Contact our team today to request a quote, discuss a custom PEG linker design, or explore our complete product catalog. Advance your PROTAC research with chemistry built for performance, reliability, and innovation.
High-Purity PEG Linkers - Ready for Your Next PROTAC Project
View the full catalog and specifications to explore our selection of high-purity PEG linkers optimized for targeted protein degradation.
FAQs
1. Why are PEG linkers widely used in PROTAC design?
PEG linkers enhance water solubility and molecular flexibility, enabling better ternary complex formation and improved drug-like properties in PROTAC molecules.
2. What are the common PEG linker lengths used?
PEG2, PEG4, PEG6, and PEG8 are the most popular options, offering tunable spacer lengths for optimal E3 ligase–target proximity.
3. Can PEG linkers improve pharmacokinetics?
Yes, PEG linkers can increase aqueous solubility and reduce non-specific binding, which contributes to better bioavailability.
References
- Mancarella C, Morrione A, Scotlandi K. PROTAC-based protein degradation as a promising strategy for targeted therapy in sarcomas[J]. International Journal of Molecular Sciences, 2023, 24(22): 16346. https://doi.org/10.3390/ijms242216346.
- Zhu Y, Dai Y, Tian Y. The Peptide PROTAC Modality: A New Strategy for Drug Discovery[J]. MedComm, 2025, 6(4): e70133. https://doi.org/10.1002/mco2.70133.
- Kubryń N, Fijałkowski Ł, Nowaczyk J, et al. PROTAC Technology as a New Tool for Modern Pharmacotherapy[J]. Molecules, 2025, 30(10): 2123. https://doi.org/10.3390/molecules30102123.
- Troup R I, Fallan C, Baud M G J. Current strategies for the design of PROTAC linkers: a critical review[J]. Exploration of Targeted Anti-tumor Therapy, 2020, 1(5): 273. https://doi.org/10.37349/etat.2020.00018.
- Distributed under Open Access license CC BY 4.0, without modification.

 Fig. 1 Schematic representation of the mechanisms of action of PROTAC and PROTAC-based genetic tools.1,5
Fig. 1 Schematic representation of the mechanisms of action of PROTAC and PROTAC-based genetic tools.1,5 Fig. 2 Flowchart of the drug development process of peptide PROTAC.2,5
Fig. 2 Flowchart of the drug development process of peptide PROTAC.2,5